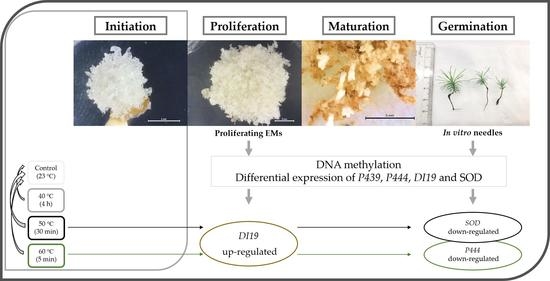
Heat Stress in Pinus halepensis Somatic Embryogenesis Induction: Effect in DNA Methylation and Differential Expression of Stress-Related Genes
Abstract
:1. Introduction
2. Results
2.1. Global DNA Methylation/Hydroxymethylation Analysis
2.2. Relative Expression of Stress-Related Genes
3. Discussion
4. Materials and Methods
4.1. SE Temperature Experiment and Plant Material Collection
4.2. Global DNA Methylation/Hydroxymethylation Analysis
4.3. Relative Expression of Stress-Related Genes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baránek, M.; Křižan, B.; Ondrušíková, E.; Pidra, M. DNA-methylation changes in grapevine somaclones following in vitro culture and thermotherapy. Plant Cell Tissue Organ. Cult. 2010, 101, 11–22. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Arnholdt-Schmitt, B. Stress-induced cell reprogramming. A role for global genome regulation? Plant Physiol. 2004, 136, 2579–2586. [Google Scholar] [CrossRef] [Green Version]
- Correia, B.; Valledor, L.; Meijón, M.; Rodriguez, J.L.; Dias, M.C.; Santos, C.; Cañal, M.J.; Rodriguez, R.; Pinto, G. Is the interplay between epigenetic markers related to the acclimation of Cork oak plants to high temperatures? PLoS ONE 2013, 8, e53543. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Seo, P.J. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 2018, 23, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Viegas, W.; Morais-Cecílio, L. Epigenetic marks in the mature pollen of Quercus suber L. (Fagaceae). Sex. Plant Reprod. 2009, 22, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lira-Medeiros, C.F.; Parisod, C.; Fernandes, R.A.; Mata, C.S.; Cardoso, M.A.; Ferreira, P.C.G. Epigenetic variation in mangrove plants occurring in contrasting natural environment. PLoS ONE 2010, 5, e10326. [Google Scholar] [CrossRef]
- Hauser, M.T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational epigenetic inheritance in plants. Biochim. Biophys. Acta Gene Regul. Mech. 2011, 1809, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smulders, M.J.M.; de Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2010, 63, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Us-Camas, R.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Peña, C. In vitro culture: An epigenetic challenge for plants. Plant Cell Tissue Organ. Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Amaral, J.; Ribeyre, Z.; Vigneaud, J.; Dia Sow, R.; Fichot, R.; Messier, C.; Pinto, G.; Nolet, P.; Maury, S. Advances and promises of epigenetics for forest trees. Forests 2020, 11, 976. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Blödner, C.; Skroppa, T.; Johnsen, Ø.; Polle, A. Freezing tolerance in two Norway spruce (Picea abies [L.] Karst.) progenies is physiologically correlated with drought tolerance. J. Plant Physiol. 2005, 162, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Montalbán, I.A.; García-Mendiguren, O.; Goicoa, T.; Ugarte, M.D.; Correia, S.; Canhoto, J.M.; Moncaleán, P. Pinus halepensis somatic embryogenesis is affected by the physical and chemical conditions at the initial stages of the process. J. For Res. 2016, 21, 143–150. [Google Scholar] [CrossRef]
- Moncaleán, P.; García-Mendiguren, O.; Novák, O.; Strnad, M.; Goicoa, T.; Ugarte, M.D.; Montalbán, I.A. Temperature and water availability during maturation affect the cytokinins and auxins profile of radiata pine somatic embryos. Front. Plant Sci. 2018, 9, 1898. [Google Scholar] [CrossRef] [Green Version]
- García-Mendiguren, O.; Montalbán, I.A.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. Environmental conditions at the initial stages of Pinus radiata somatic embryogenesis affect the production of somatic embryos. Trees Struct. Funct. 2016, 30, 949–958. [Google Scholar] [CrossRef]
- Yakovlev, I.A.; Carneros, E.; Lee, Y.K.; Olsen, J.E.; Fossdal, C.G. Transcriptional profiling of epigenetic regulators in somatic embryos during temperature induced formation of an epigenetic memory in Norway spruce. Planta 2016, 243, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Castander-Olarieta, A.; Montalbán, I.A.; De Medeiros Oliveira, E.; Dell’aversana, E.; D’amelia, L.; Carillo, P.; Steiner, N.; Fraga, H.P.D.F.; Guerra, M.P.; Goicoa, T.; et al. Effect of thermal stress on tissue ultrastructure and metabolite profiles during initiation of radiata pine somatic embryogenesis. Front Plant Sci. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Valledor, L.; Jorrín, J.V.; Rodríguez, J.L.; Lenz, C.; Meijón, M.; Rodríguez, R.; Cañal, M.J. Combined proteomic and transcriptomic analysis identifies differentially expressed pathways associated to Pinus radiata needle maturation. J. Proteome Res. 2010, 9, 3954–3979. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, Ø.; Fossdal, C.G.; Nagy, N.; MØlmann, J.; Dæhlen, O.G.; SkrØppa, T. Climatic adaptation in Picea abies progenies is affected by the temperature during zygotic embryogenesis and seed maturation. Plant Cell Environ. 2005, 28, 1090–1102. [Google Scholar] [CrossRef]
- Von Arnold, S.; Clapham, D.; Abrahamsson, M. Embryology in Conifers. In Advances in Botanical Research, 1st ed.; Cánovas, F.N., Ed.; Elsevier: Cambridge, MA, USA, 2019; Volume 89, pp. 157–184. [Google Scholar]
- Montalbán, I.A.; Setién-Olarra, A.; Hargreaves, C.L.; Moncaleán, P. Somatic embryogenesis in Pinus halepensis Mill.: An important ecological species from the Mediterranean forest. Trees 2013, 27, 1339–1351. [Google Scholar] [CrossRef]
- Pereira, C.; Castander-Olarieta, A.; Montalbán, I.A.; Pěnčík, A.; Petřík, I.; Pavlović, I.; Oliveira, E.D.M.; Freitas Fraga, H.P.d.; Guerra, M.P.; Novák, O.; et al. Embryonal masses induced at high temperatures in Aleppo pine: Cytokinin profile and cytological characterization. Forests 2020, 11, 807. [Google Scholar] [CrossRef]
- Feher, A.; Ötvös, K.; Pasternak, T.P.; Pettkó-Szandtner, A. The involvement of reactive oxygen species (ROS) in the cell cycle activation (G 0 -to-G 1 transition) of plant cells. Plant Signal Behav. 2008, 3, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, F.C.; Zhang, W.Z.; Song, L.F.; Wu, W.H.; Chen, Y.F. Arabidopsis Di19 functions as a transcription factor and modulates PR1, PR2, and PR5 expression in response to drought stress. Mol. Plant 2013, 6, 1487–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, C.; Valledor, L.; Sáez, P.; Hasbún, R.; Sánchez-Olate, M.; Cañal, M.J.; Ríos, D. Changes in gene expression in needles and stems of Pinus radiata rootstock plants of different ontogenic age. Amer. J. Plant Sci. 2016, 07, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Neilson, K.A.; Gayani Gammulla, C.; Mirzaei, M.; Imin, N.; Haynes, P.A. Proteomic analysis of temperature stress in plants. Wiley Online Libr. 2010, 10, 828–845. [Google Scholar] [CrossRef]
- Ling, Y.; Serrano, N.; Gao, G.; Atia, M.; Mokhtar, M.; Woo, Y.H.; Bazin, J.; Veluchamy, A.; Benhamed, M.; Crespi, M.; et al. Thermopriming triggers splicing memory in Arabidopsis. J. Exp. Bot. 2018, 69, 2659–2675. [Google Scholar] [CrossRef] [Green Version]
- Dia-Sow, M.; Allona, I.; Ambroise, C.; Conde, D.; Fichot, R.; Gribkova, S.; Jorge, V.; Le-Provost, G.; Pâques, L.; Plomion, C.; et al. Epigenetics in forest trees: State of the art and potential implications for breeding and management in a context of climate change. Adv. Bot. Res. 2018, 88, 387–453. [Google Scholar] [CrossRef]
- Boyko, A.; Kovalchuk, I. Epigenetic control of plant stress response. Environ. Mol. Mutag. 2008, 49, 61–72. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Viejo, M.; Santamaría, M.E.; Rodríguez, J.L.; Valledor, L.; Meijón, M.; Pérez, M.; Pascual, J.; Hasbún, R.; Fraga, M.F.; Berdasco, M.; et al. Epigenetics, the role of DNA methylation in tree development. Methods Mol. Biol. 2012, 877, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumari, R.; Sharma, V.; Sharma, V. Roles, and establishment, maintenance and erasing of the epigenetic cytosine methylation marks in plants. J. Genet. 2013, 92, 629–666. [Google Scholar] [CrossRef] [PubMed]
- Korotko, U.; Chwiałkowska, K.; Sańko-Sawczenko, I.; Kwasniewski, M. DNA demethylation in response to heat stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 1555. [Google Scholar] [CrossRef]
- Gallusci, P.; Dai, Z.; Génard, M.; Gauffretau, A.; Leblanc-Fournier, N.; Richard-Molard, C.; Vile, D.; Brunel-Muguet, S. Epigenetics for plant improvement: Current knowledge and modeling avenues. Trends Plant Sci. 2017, 22, 610–623. [Google Scholar] [CrossRef]
- Noceda, C.; Salaj, T.; Pérez, M.; Viejo, M.; Cañal, M.J.; Salaj, J.; Rodriguez, R. DNA demethylation and decrease on free polyamines is associated with the embryogenic capacity of Pinus nigra Arn. cell culture. Trees 2009, 23, 1285–1293. [Google Scholar] [CrossRef]
- Amaral-Silva, P.; Clarindo, W.; Guilhen, J.; de Jesus Passos, A.; Sanglard, N.; Ferreira, A. Global 5-methylcytosine and physiological changes are triggers of indirect somatic embryogenesis in Coffea canephora. Protoplasma 2021, 258, 45–57. [Google Scholar] [CrossRef]
- De-la-Peña, C.; Nic-Can, G.I.; Galaz-Ávalos, R.M.; Avilez-Montalvo, R.; Loyola-Vargas, V.M. The role of chromatin modifications in somatic embryogenesis in plants. Front Plant Sci. 2015, 6, 635. [Google Scholar] [CrossRef] [Green Version]
- Bravo, S.; Bertín, A.; Turner, A.; Sepúlveda, F.; Jopia, P.; Parra, M.J.; Castillo, R.; Hasbún, R. Differences in DNA methylation, DNA structure and embryogenesis-related gene expression between embryogenic and non-embryogenic lines of Pinus radiata D. don. Plant Cell Tissue Organ. Cult. 2017, 130, 521–529. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Pereira, C.; Sales, E.; Meijón, M.; Arrillaga, I.; Cañal, M.J.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P.; Montalbán, I.A. Induction of radiata pine somatic embryogenesis at high temperatures provokes a long-term decrease in dna methylation/hydroxymethylation and differential expression of stress-related genes. Plants 2020, 9, 1762. [Google Scholar] [CrossRef]
- Johnsen, Ø.; Dæhlen, O.G.; Østreng, G.; Skrøppa, T. Daylength and temperature during seed production interactively affect adaptive performance of Picea abies progenies. New Phytol. 2005, 168, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.; Blevins, T.; Yao, Y.; Golubov, A.; Bilichak, A.; Ilnytskyy, Y.; Hollander, J.; Meins, F., Jr.; Kovalchuk, I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-Like proteins. PLoS ONE 2010, 5, e9514. [Google Scholar] [CrossRef]
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Marcos, J.G.; Fluch, S.; Fraga, M.F.; Guevara, M.Á.; Abarca, D.; et al. Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol. Evol. 2013, 3, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Valledor, L.; Hasbún, R.; Meijón, M.; Rodríguez, J.L.; Santamaría, E.; Viejo, M.; Berdasco, M.; Feito, I.; Fraga, M.F.; Cañal, M.J.; et al. Involvement of DNA methylation in tree development and micropropagation. Plant Cell Tissue Organ. Cult. 2007, 91, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Fraga, M.F.; Cañal, M.; Rodríguez, R. Phase-change related epigenetic and physiological changes in Pinus radiata D. Don. Planta 2002, 215, 672–678. [Google Scholar] [CrossRef]
- Shi, D.; Ali, I.; Tang, J.; Yang, W. New insights into 5hmC DNA modification: Generation, distribution and function. Front. Genet. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Yakovlev, I.A.; Gackowski, D.; Abakir, A.; Viejo, M.; Ruzov, A.; Olinski, R.; Starczak, M.; Fossdal, C.G.; Krutovsky, K.V. Mass spectrometry reveals the presence of specific set of epigenetic DNA modifications in the Norway spruce genome. Sci. Rep. 2019, 9, 19314. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Escandón, M.; Cañal, M.J.; Pascual, J.; Pinto, G.; Correia, B.; Amaral, J.; Meijón, M. Integrated physiological and hormonal profile of heat-induced thermotolerance in Pinus radiata. Tree Physiol. 2015, 36, 63–77. [Google Scholar] [CrossRef]
- Milla, M.A.; Townsend, J.; Chang, I.F.; Cushman, J.C. The Arabidopsis AtDi19 gene family encodes a novel type of Cys2/His2 zinc-finger protein implicated in ABA-independent dehydration, high-salinity stress and light signaling pathways. Plant Mol. Biol. 2006, 61, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, J.; Shi, W.; Cao, X.; Luo, J.; Polle, A.; Luo, Z.B. Comparative transcriptomic analysis reveals the roles of overlapping heat-/drought-responsive genes in poplars exposed to high temperature and drought. Sci. Rep. 2017, 7, 43215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yu, C.; Chen, C.; He, C.; Zhu, Y.; Huang, W. Identification of rice Di19 family reveals OsDi19—4 involved in drought resistance. Plant Cell Rep. 2014, 33, 2047–2062. [Google Scholar] [CrossRef]
- Qin, L.-X.; Li, Y.; Li, D.-D.; Xu, W.-L.; Zheng, Y.; Li, X.-B. Arabidopsis drought-induced protein Di19-3 participates in plant response to drought and high salinity stresses. Plant Mol. Biol. 2014, 86, 609–625. [Google Scholar] [CrossRef]
- Efeoǧlu, B. Heat shock proteins and heat shock response in plants. Gazi. Univ. J. Sci. 2009, 22, 67–75. [Google Scholar]
- Pérez-Oliver, M.A.; Haro, J.G.; Pavlović, I.; Novák, O.; Segura, J.; Sales, E.; Arrillaga, I. Priming maritime pine megagametophytes during somatic embryogenesis improved plant adaptation to heat stress. Plants 2021, 10, 446. [Google Scholar] [CrossRef]
- Escandón, M.; Valledor, L.; Pascual, J.; Pinto, G.; Cañal, M.J.; Meijón, M. System-wide analysis of short-term response to high temperature in Pinus radiata. J. Exp. Bot. 2017, 68, 3629–3641. [Google Scholar] [CrossRef]
- Dobrá, J.; Černý, M.; Štorchová, H.; Dobrev, P.; Skalák, J.; Jedelský, P.L.; Lukšanová, H.; Gaudinová, A.; Pešek, B.; Malbecka, J.; et al. The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis. Plant Sci. 2015, 231, 52–61. [Google Scholar] [CrossRef]
- Montalbán, I.A.; García-Mendiguren, O.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. Cold storage of initial plant material affects positively somatic embryogenesis in Pinus radiata. New For. 2015, 46, 309–317. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Plantlet regeneration via somatic embryogenesis from subcultured callus of mature embryos of Picea abies (Norway spruce). Vitr. Cell. Dev. Biol. 1986, 22, 685–688. [Google Scholar] [CrossRef]
- Walter, C.; Find, J.I.; Grace, L.J. Somatic embryogenesis and genetic transformation in Pinus radiata. In Protocol for Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 11–24. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Improved media for in vitro culture of Prunus sp. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Singh, A.P.; Davies, H. Multiplication of meristematic tissue: A new tissue culture system for radiata pine. In Genetic Manipulation of Woody Plants; Hanover, J.W., Keathley, D.E., Wilson, C.M., Kuny, G., Eds.; Springer: Boston, MA, USA, 1988; pp. 413–432. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| ID | Name | Forward (5′ → 3′) | Reverse (5′ → 3′) | Tm (°C) |
|---|---|---|---|---|
| ACT | ACTIN | CACTGCACTTGCTCCCAGTA | AACCTCCGATCCAAACACTG | 60 |
| P439 | CHLOROPLAST SMALL HEAT PROTEIN | AAGTTGTCGGTTCGAACCCC | CAGAACACCGTCCTCCACAG | 62 |
| P444 | HSP20 FAMILY PROTEIN | TTTCCGACTTCTTCACGGGG | TTTGACAGTCCCGGCATGTC | 62 |
| DI19 | DEHIDRATION INDUCED PROTEIN 19 | ATAGATGCCCATGCTGTGTAG | CTTCCCTCTGTTCCCACTTG | 54 |
| SOD | SUPEROXIDE DISMUTASE [Cu–Zn] | ACAAAACGGGTGCATGTCAAC | CCCATCCGCTCCTACAGTTAC | 66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.; Castander-Olarieta, A.; Sales, E.; Montalbán, I.A.; Canhoto, J.; Moncaleán, P. Heat Stress in Pinus halepensis Somatic Embryogenesis Induction: Effect in DNA Methylation and Differential Expression of Stress-Related Genes. Plants 2021, 10, 2333. https://doi.org/10.3390/plants10112333
Pereira C, Castander-Olarieta A, Sales E, Montalbán IA, Canhoto J, Moncaleán P. Heat Stress in Pinus halepensis Somatic Embryogenesis Induction: Effect in DNA Methylation and Differential Expression of Stress-Related Genes. Plants. 2021; 10(11):2333. https://doi.org/10.3390/plants10112333
Chicago/Turabian StylePereira, Cátia, Ander Castander-Olarieta, Ester Sales, Itziar A. Montalbán, Jorge Canhoto, and Paloma Moncaleán. 2021. "Heat Stress in Pinus halepensis Somatic Embryogenesis Induction: Effect in DNA Methylation and Differential Expression of Stress-Related Genes" Plants 10, no. 11: 2333. https://doi.org/10.3390/plants10112333
APA StylePereira, C., Castander-Olarieta, A., Sales, E., Montalbán, I. A., Canhoto, J., & Moncaleán, P. (2021). Heat Stress in Pinus halepensis Somatic Embryogenesis Induction: Effect in DNA Methylation and Differential Expression of Stress-Related Genes. Plants, 10(11), 2333. https://doi.org/10.3390/plants10112333