Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT
Abstract
:1. Introduction
2. Results
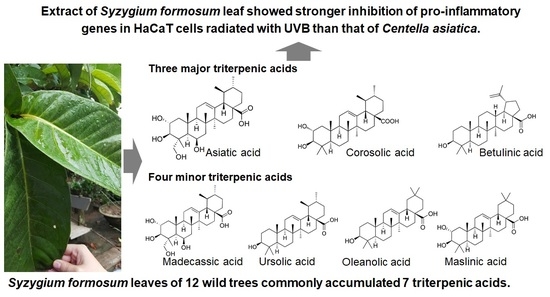
2.1. Triterpenic Acid Compositions of 12 Wild S. formosum Trees
2.1.1. Variations of Triterpenic Acids
2.1.2. Comparisons of Triterpenic Acids with Centella asiatica
2.2. Antioxidative Activities and Total Phenolics
2.3. Anti-Inflammatory Effects
2.3.1. mRNA Expression of Pro-Inflammatory Genes
2.3.2. Protein Levels of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Plant Samples and Reagents
4.2. Extraction of Plants
4.3. Instrumental Analyses of Triterpenic Acids
4.4. Chemical and Biological Activity Assay
4.4.1. Antioxidative Activity
4.4.2. Total Phenolic Compounds
4.4.3. Cell Culture and Viability Assay
4.4.4. Anti-Inflammatory Effects of Plant Extracts
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hashim, P.; Sidek, H.; Helan, M.H.M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene composition and bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella asiatica in dermatology: An overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef]
- An, I.-S.; An, S.; Choe, T.-Β.; Kang, S.-Μ.; Lee, J.H.; Park, I.-C.; Jin, Y.-W.; Lee, S.-J.; Bae, S. Centella asiatica protects against UVB-induced HaCaT keratinocyte damage through microRNA expression changes. Int. J. Mol. Med. 2012, 30, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.; Lee, J.-A.; Shin, S.; Roh, K.-B.; Kim, J.-H.; Park, D. Madecassoside inhibits melanin synthesis by blocking ultraviolet-induced inflammation. Molecules 2013, 18, 15724–15736. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.J.; Bae, S.; Kim, K.; An, I.S.; Ahn, K.J.; An, S.; Cha, H.J. Asiaticoside, a component of Centella asiatica, inhibits melanogenesis in B16F10 mouse melanoma. Mol. Med. Rep. 2014, 10, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kim, H.-L.; Lee, M.H.; You, K.E.; Kwon, B.-J.; Seo, H.J.; Park, J.-C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Lomunova, M.; Vu, T.P.D.; Le, B.V.; Kim, Y.H.; Kang, J.S.; Hwang, I. Anti-allergic effects of the ethanol extract of Syzygium formosum (Wall.) Masam leaves and its immunoregulatory mechanisms. J. Ethnopharmacol. 2018, 211, 171–179. [Google Scholar] [CrossRef]
- Vu, T.P.D.; Khong, T.Q.; Nguyen, T.M.N.; Kim, Y.H.; Kang, J.S. Phytochemical profile of Syzygium formosum (Wall.) Masam leaves using HPLC–PDA–MS/MS and a simple HPLC–ELSD method for quality control. J. Pharm. Biomed. Anal. 2019, 168, 1–12. [Google Scholar]
- Lee, Y.S.; Jin, D.-Q.; Beak, S.-M.; Lee, E.-S.; Kim, J.-A. Inhibition of ultraviolet-A-modulated signaling pathways by asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur. J. Pharmacol. 2003, 476, 173–178. [Google Scholar]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef]
- Muthusamy, V.; Piva, T.J. A comparative study of UV-induced cell signalling pathways in human keratinocyte-derived cell lines. Arch. Dermatol. Res. 2013, 305, 817–833. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Li, W.-B.; Zhou, J.; Liu, X.; Wang, L.; Chen, B.; Wang, M.-K.; Ji, L.; Hu, W.-C.; Li, F. Oleanane-and ursane-type triterpene saponins from Centella asiatica exhibit neuroprotective effects. J. Agric. Food Chem. 2020, 68, 6977–6986. [Google Scholar] [CrossRef]
- Sirignano, C.; Nadembega, P.; Poli, F.; Romano, B.; Lucariello, G.; Rigano, D.; Taglialatela-Scafati, O. Triterpenoids from Vitellaria paradoxa stem barks reduce nitrite levels in LPS-stimulated macrophages. Plants 2021, 10, 1006. [Google Scholar] [CrossRef]
- Yang, J.; Leng, J.; Li, J.-J.; Tang, J.-f.; Li, Y.; Liu, B.-L.; Wen, X.-D. Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine 2016, 23, 181–190. [Google Scholar] [CrossRef]
- Lo, W.-L.; Hsu, T.-I.; Yang, W.-B.; Kao, T.-J.; Wu, M.-H.; Huang, Y.-N.; Yeh, S.-H.; Chuang, J.-Y. Betulinic acid-mediated tuning of PERK/CHOP signaling by Sp1 inhibition as a novel therapeutic strategy for glioblastoma. Cancers 2020, 12, 981. [Google Scholar] [CrossRef]
- Chianese, G.; Masi, F.; Cicia, D.; Ciceri, D.; Arpini, S.; Falzoni, M.; Pagano, E.; Taglialatela-Scafati, O. Isomadecassoside, a new ursane-type triterpene glycoside from Centella asiatica leaves, reduces nitrite levels in LPS-stimulated macrophages. Biomolecules 2021, 11, 494. [Google Scholar] [CrossRef]
- Clausen, M.-L.; Kezic, S.; Olesen, C.; Agner, T. Cytokine concentration across the stratum corneum in atopic dermatitis and healthy controls. Sci. Rep. 2020, 10, 21895. [Google Scholar] [CrossRef]
- Lyubchenko, T.; Collins, H.K.; Goleva, E.; Leung, D.Y. Skin tape sampling technique identifies proinflammatory cytokines in atopic dermatitis skin. Ann. Allergy Asthma Immunol. 2021, 126, 46–53.e42. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Kim, J.; Vaish, V.; Feng, M.; Field, K.; Chatzistamou, I.; Shim, M. Transgenic expression of cyclooxygenase-2 (COX2) causes premature aging phenotypes in mice. Aging 2016, 8, 2392. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.F.O.; Barbosa-Filho, J.M.; de Azevedo Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.P.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.d.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, M.-H. Melatonin and polyphenol contents in some edible sprouts (alfalfa, chicory, rape, red kale and sunflower). Prev. Nutr. Food Sci. 2011, 16, 184–188. [Google Scholar] [CrossRef] [Green Version]







| S. formosum | Triterpenic Acids a (mg/g Dry Weight) | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Madecassic Acid | Asiatic Acid | Maslinic Acid | Corosolic Acid | Betulinic Acid | Oleanolic Acid | Ursolic Acid | ||
| S1 | 0.20 ± 0.02 | 3.36 ± 0.51 | 0.26 ± 0.01 | 0.89 ± 0.15 | 4.74 ± 0.84 | 0.065 ± 0.005 | 0.18 ± 0.01 | 9.695 ± 1.545 |
| S2 | 0.67 ± 0.01 | 5.26 ± 0.25 | 0.53 ± 0.02 | 1.59 ± 0.12 | 3.09 ± 0.02 | 0.10 ± 0.01 | 0.23 ± 0.01 | 11.44 ± 0.39 |
| S3 | 0.50 ± 0.01 | 5.57 ± 0.17 | 0.41 ± 0.01 | 1.25 ± 0.06 | 4.54 ± 0.45 | 0.12 ± 0.01 | 0.28 ± 0.01 | 12.65 ± 0.68 |
| S4 | 0.98 ± 0.04 | 12.6 ± 0.5 | 1.04 ± 0.05 | 3.54 ± 0.04 | 5.27 ± 0.36 | 0.33 ± 0.2 | 0.81 ± 0.04 | 24.56 ± 1.05 |
| S5 | 0.37 ± 0.02 | 5.89 ± 0.02 | 0.98 ± 0.01 | 3.76 ± 0.12 | 3.89 ± 0.02 | 0.26 ± 0.01 | 0.66 ± 0.01 | 15.81 ± 0.18 |
| S6 | 0.25 ± 0.04 | 2.78 ± 0.29 | 0.44 ± 0.04 | 1.64 ± 0.22 | 4.23 ± 0.43 | 0.065 ± 0.005 | 0.16 ± 0.01 | 9.56 ± 1.03 |
| S7 | 0.24 ± 0.02 | 3.99 ± 0.05 | 0.37 ± 0.04 | 1.35 ± 0.07 | 2.53 ± 0.24 | 0.08 ± 0.01 | 0.23 ± 0.02 | 8.77 ± 0.43 |
| S8 | 0.44 ± 0.03 | 4.68 ± 0.54 | 0.84 ± 0.06 | 3.78 ± 0.42 | 5.11 ± 0.50 | 0.18 ± 0.01 | 0.52 ± 0.05 | 15.53 ± 1.59 |
| S9 | 0.29 ± 0.01 | 6.81 ± 0.46 | 0.95 ± 0.03 | 3.83 ± 0.30 | 3.07 ± 0.45 | 0.22 ± 0.02 | 0.66 ± 0.03 | 15.81 ± 1.18 |
| S10 | 0.39 ± 0.03 | 4.51 ± 0.80 | 0.84 ± 0.05 | 4.02 ± 0.70 | 5.89 ± 0.78 | 0.19 ± 0.02 | 0.60 ± 0.03 | 16.41 ± 2.38 |
| S11 | 0.27 ± 0.02 | 7.76 ± 0.61 | 0.86 ± 0.01 | 3.66 ± 0.47 | 2.70 ± 0.25 | 0.19 ± 0.02 | 0.56 ± 0.01 | 15.99 ± 1.35 |
| S12 | 0.82 ± 0.01 | 10.07 ± 0.09 | 2.16 ± 0.02 | 6.21 ± 0.01 | 6.05 ± 0.22 | 0.53 ± 0.03 | 1.36 ± 0.02 | 27.19 ± 0.26 |
| Average | 0.42 ± 0.25 | 5.84 ± 2.81 | 0.79 ± 0.47 | 2.95 ± 1.50 | 4.24 ± 1.20 | 0.19 ± 0.12 | 0.51 ± 0.32 | 14.94 ± 5.50 |
| C. asiatica | 1.72 ± 0.26 | 3.21 ± 0.7 | NA b | NA | NA | NA | NA | 4.94 ± 0.96 |
| Gene | Forward | Reverse |
|---|---|---|
| IL-1β | GGG CCT CAA GGA AAA GAA TC | TTC TGC TTG AGA GGT GCT GA |
| IL-6 | AGG AGA CTT GCC TGG TGA AA | CAG GGG TGG TTA TTG CAT CT |
| IL-8 | TCT GTG TGA AGG TGC ACT T | AGC CCT CTT CAA AAA CTT CT |
| COX-2 | GGT CTG GTG CCT GGT CTG ATG | GTC CTT TCA AGG AGA ATG GTG C |
| Actin | GGC GGA CTA TGA CTT AGT TG | AAC AAC AAT GTG CAA TCA A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-a.; Kim, M.Y.; Lee, N.-Y.; Lim, J.; Park, K.-b.; Lee, C.-K.; Nguyen, V.D.; Kim, J.; Park, J.-T.; Park, J.-I. Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT. Plants 2021, 10, 2428. https://doi.org/10.3390/plants10112428
Park H-a, Kim MY, Lee N-Y, Lim J, Park K-b, Lee C-K, Nguyen VD, Kim J, Park J-T, Park J-I. Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT. Plants. 2021; 10(11):2428. https://doi.org/10.3390/plants10112428
Chicago/Turabian StylePark, Hyun-ah, Mi Yoon Kim, Nan-Young Lee, Jaeyoon Lim, Kyu-been Park, Chang-Kyu Lee, Van Dao Nguyen, Jaehan Kim, Jong-Tae Park, and Jong-Il Park. 2021. "Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT" Plants 10, no. 11: 2428. https://doi.org/10.3390/plants10112428
APA StylePark, H. -a., Kim, M. Y., Lee, N. -Y., Lim, J., Park, K. -b., Lee, C. -K., Nguyen, V. D., Kim, J., Park, J. -T., & Park, J. -I. (2021). Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT. Plants, 10(11), 2428. https://doi.org/10.3390/plants10112428