Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative
Abstract
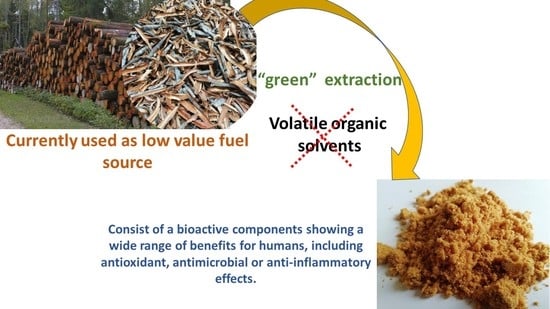
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Sample Preparation
- Only with organic solvents increasing their polarity, n-hexane→ethyl acetate→ethanol: water according to the previously developed methodology described in [11];
- Only with deionized water: tap water and water with different pH;
- Combined extraction methods: with deionized water and then with organic solvents sequential.
3.4. Total Polyphenol Content (TPC)
3.5. Antioxidant Activity
3.6. Total Proanthocyanidin Content
3.7. Energy Consumption Measurements
3.8. Statistical Treatment of the Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aspé, E.; Fernández, K. Comparison of phenolic extracts obtained of Pinus radiata bark from pulp and paper industry and sawmill industry. Maderas. Cienc. y Tecnol. 2011, 13, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Mantia, A.L.; Pappalardo, F.; Ragusa, M.; Renis, M.; Di Giacomo, C. The Antioxidant Activities of Betula etnensis Rafin. Ethanolic Extract Exert Protective and Anti-diabetic Effects on Streptozotocin-Induced Diabetes in Rats. Antioxidants 2020, 9, 847. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidaković, V.; Novaković, M.; Popović, Z.; Janković, M.; Matić, R.; Tešević, V.; Bojović, S. Significance of diarylheptanoids for chemotaxonomical distinguishing between Alnus glutinosa and Alnus incana. Holzforschung 2018, 72, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Klaric, M.; Oven, P.; Gorišek, Ž.; Španic, N.; Pervan, S. Yield of Stirred Cold Maceration and Extraction of Milled European Black Alder Wood and Bark using Different Solvents. BioResources 2016, 11, 9244–9254. [Google Scholar] [CrossRef] [Green Version]
- Novaković, M.; Stanković, M.; Vučković, I.; Todorović, N.; Trifunović, S.; Tešević, V.; Vajs, V.; Milosavljević, S. Diarylheptanoids from Alnus glutinosa Bark and Their Chemoprotective Effect on Human Lymphocytes DNA. Planta Med. 2013, 79, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the antioxidant activity of Betula pendula leaves extract and its effects on model foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef]
- Lee, S.; Oh, D.-G.; Singh, D.; Lee, H.J.; Kim, G.R.; Lee, S.; Lee, J.S.; Lee, C.H. Untargeted Metabolomics Toward Systematic Characterization of Antioxidant Compounds in Betulaceae Family Plant Extracts. Metabolites 2019, 9, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sati, S.; Sati, O.; Sati, N. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; He, T.; Chang, Y.; Zhao, Y.; Chen, X.; Bai, S.; Wang, L.; Shen, M.; She, G. The Genus Alnus, A Comprehensive Outline of Its Chemical Constituents and Biological Activities. Molecules 2017, 22, 1383. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, D.; Desseva, I.; Stoyanova, M.; Petkova, N.; Terzyiska, M.; Lante, A. Impact of In Vitro Gastrointestinal Digestion on the Bioaccessibility of Phytochemical Compounds from Eight Fruit Juices. Molecules 2021, 26, 1187. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Stefanello, F.S.; Huerta, K.D.M.; Monteiro, S.S.; da Rosa, C.S.; Terra, N.N. Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit ( Actinidia deliciosa ). Food Chem. 2016, 199, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Bennett, L.E.; Jegasothy, H.; Konczak, I.; Frank, D.; Sudharmarajan, S.; Clingeleffer, P.R. Total polyphenolics and anti-oxidant properties of selected dried fruits and relationships to drying conditions. J. Funct. Foods 2011, 3, 115–124. [Google Scholar] [CrossRef]
- ZY, J.; LR, H. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- F, C.; MA, V.; G, C. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Lauberts, M.; Telysheva, G.; Venskutonis, P.R.; Lauberte, L.; Dizhbite, T.; Kazernavičiūte, R.; Pukalskas, A. Diarylheptanoid-rich extract of grey and black alder barks: An effective dietary antioxidant in mayonnaise. Chem. Pap. 2017. [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; El Zerey-Belaskri, A.; Gismondi, A.; Di Marco, G.; Canini, A.; Bechlaghem, N.; Atik Bekkara, F.; Djebli, N. Influence of plant and environment parameters on phytochemical composition and biological properties of Pistacia atlantica Desf. Biochem. Syst. Ecol. 2021, 95, 104231. [Google Scholar] [CrossRef]
- Agarwal, C.; Hofmann, T.; Visi-Rajczi, E.; Pásztory, Z. Low-frequency, green sonoextraction of antioxidants from tree barks of Hungarian woodlands for potential food applications. Chem. Eng. Process. Process Intensif. 2021, 159, 108221. [Google Scholar] [CrossRef]
- Skrypnik, L.; Grigorev, N.; Michailov, D.; Antipina, M.; Danilova, M.; Pungin, A. Comparative study on radical scavenging activity and phenolic compounds content in water bark extracts of alder (Alnus glutinosa (L.) Gaertn.), oak (Quercus robur L.) and pine (Pinus sylvestris L.). Eur. J. Wood Wood Prod. 2019, 77, 879–890. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852. [Google Scholar] [CrossRef] [Green Version]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Vieito, C.; Fernandes, É.; Velho, M.V.; Pires, P. The effect of different solvents on extraction yield, total phenolic content and antioxidant activity of extracts from pine bark (Pinus pinaster subsp. atlantica). Chem. Eng. Trans. 2018, 64, 127–132. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]










| Used Solvent | Yield, % | TPC GAE g/g | PAC % | DPPH• IC50 mg/L |
|---|---|---|---|---|
| Tap water * | 23.31 ± 0.44 | 0.56 ± 0.012 | 13.42 ± 0.23 | 6.79 ± 0.15 |
| Deionized water with Na2CO3, pH = 10.92 | 22.20 ± 0.39 | 0.56 ± 0.014 | 14.25 ± 0.25 | 7.12 ± 0.18 |
| Electrolyzed water, pH = 11.62 | 22.87 ± 0.36 | 0.56 ± 0.014 | 14.28 ± 0.37 | 7.17 ± 0.14 |
| Electrolyzed water, pH = 2.57 | 22.13 ± 0.32 | 0.58 ± 0.018 | 11.21 ± 0.28 | 6.26 ± 0.10 |
| Deionized, water | 23.53 ± 0.41 | 0.63 ± 0.02 | 21.59 ± 0.42 | 5.18 ± 0.11 |
| Kruskal–Wallis test | ||||
| p value | 0.332 | 0.1902 | 0.2164 | 0.2137 |
| Solvent | Reference Yield, % | After Deionized Water Extraction, % | Organic Soluble Part in Deionized Water, % |
|---|---|---|---|
| n-hexane | 2.45 ± 0.05 | 1.77 ± 0.04 | 27.80 ± 0.81 |
| Ethyl acetate | 10.95 ± 0.24 | 0.92 ± 0.017 | 91.60 ± 2.19 |
| 40% (v:v) ethanol | 16.41 ± 0.36 | 2.35 ± 0.06 | 85.68 ± 2.37 |
| Additive | Concentration of Additive, w/w, mg/g | Induction Period (IP), h | Protection Factor (PF) |
|---|---|---|---|
| Blank | - | 2.32 | 1.00 |
| Water extract | 1.00 | 3.26 | 1.41 |
| Water extract | 5.00 | 4.78 | 2.06 |
| Temperature, °C | Total Energy Consumption, Wh | Specific Energy Consumption, Wh/g | Total Extraction Time, s |
|---|---|---|---|
| 70 | 97.99 ± 2.54 | 13.21 ± 0.27 | 2315 |
| 90 | 131.19 ± 3.15 | 14.96 ± 0.36 | 2379 |
| 110 | 160.77 ± 4.66 | 16.96 ± 0.39 | 2556 |
| 130 | 207.33 ± 4.35 | 20.79 ± 0.54 | 2631 |
| 150 | 238.52 ± 6.21 | 20.51 ± 0.48 | 2697 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauberts, M.; Pals, M. Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants 2021, 10, 2531. https://doi.org/10.3390/plants10112531
Lauberts M, Pals M. Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants. 2021; 10(11):2531. https://doi.org/10.3390/plants10112531
Chicago/Turabian StyleLauberts, Maris, and Matiss Pals. 2021. "Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative" Plants 10, no. 11: 2531. https://doi.org/10.3390/plants10112531
APA StyleLauberts, M., & Pals, M. (2021). Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants, 10(11), 2531. https://doi.org/10.3390/plants10112531