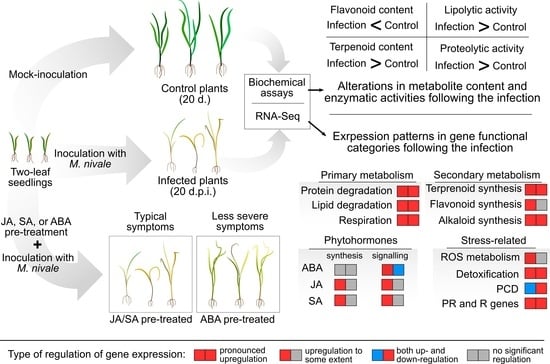
Alterations in the Transcriptome of Rye Plants following the Microdochium nivale Infection: Identification of Resistance/Susceptibility-Related Reactions Based on RNA-Seq Analysis
Abstract
:1. Introduction
2. Results
2.1. Modulation of Gene Expression in Rye Plants Following M. nivale Infection
2.1.1. The Cell Wall Category
2.1.2. The Primary Metabolism Category
2.1.3. The Secondary Metabolism Category
2.1.4. The Stress-Related Category
2.1.5. The Signaling-Related Category
2.1.6. The Transport-Related Category
2.2. Alterations in Lipase in Protease Activities, Terpenoid and Flavonoid Content in Rye Plants Following M. nivale Infection
2.3. The Effect of Exogenous Phytohormones on M. nivale Infection
3. Discussion
4. Methods
4.1. Plant and Fungi Growth Conditions and Plant Inoculation
4.2. The Evaluation of the Symptom Manifestation
4.3. Treatment with Exogenous Phytohormones
4.4. Estimation of Total Terpenoid Content
4.5. Estimation of Total Flavonoid Content
4.6. Lipolytic and Proteolytic Activity Measurement
4.7. RNA Isolation, Sequencing
4.8. The Analysis of Differentially Expressed Genes
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamond, H.; Cooke, B.M.; Dunne, B. Information note: Microdochium leaf blotch of oats—a new foliar disease caused by Microdochium nivale var. nivale in Ireland. Plant Var. Seeds 1995, 8, 171–174. [Google Scholar]
- Tronsmo, A.M.; Hsiang, T.; Okuyama, H.; Nakajima, T. Low Temperature Plant Microbe Interactions under Snow; Iriki, N., Gaudet, D.A., Tronsmo, A.M., Matsumoto, N., Yoshida, M., Nishimune, A., Eds.; Hokkaido National Agricultural Experiment Station: Hokkaido, Japan, 2001; Chapter 7.
- Turner, A.S.; Nicholson, P.; Edwards, S.G.; Bateman, G.L.; Morgan, L.W.; Todd, A.D.; Parry, D.W.; Marshall, J.; Nuttall, M. Relationship between brown foot rot and DNA of Microdochium nivale, determined by quantitative PCR, in stem bases of winter wheat. Plant Pathol. 2002, 51, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, N. Snow molds: A group of fungi that prevail under snow. Microbes Environ. 2009, 24, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagkaeva, T.Y.; Orina, A.S.; Gavrilova, O.P.; Gogina, N.N. Evidence of Microdochium fungi associated with cereal grains in Russia. Microorganisms 2020, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Sheshegova, T.K. Analysis of a phytosanitary condition of sowings of spring grain crops in the Kirov region (Analytical review). Agr. Sci. Euro-North-East 2015, 48, 10–14. [Google Scholar] [CrossRef]
- Utkina, E.I.; Kedrova, L.I.; Parfyonova, E.S.; Shamova, M.G. Influence of snow mold on winter rye productivity in the Kirov region. Agr. Sci. Euro-North-East 2019, 20, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Ponomareva, M.L.; Gorshkov, V.Y.; Ponomarev, S.N.; Korzun, V.; Miedaner, T. Snow mold of winter cereals: A complex disease and a challenge for resistance breeding. Theor. Appl. Genet. 2021, 134, 419–433. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chang, S.-W.; Jung, G.-H. Response of bentgrass cultivars to Microdochium nivale isolates collected from golf courses. Plant Pathol. J. 2011, 27, 232–341. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-H. Difference of susceptibility on bentgrass cultivars to pink snow mold caused by Microdochium nivale. Asian J. Turfgrass Sci. 2011, 25, 177–183. [Google Scholar]
- Gołębiowska-Pikania, G.; Dziurka, M.; Wąsek, I.; Wajdzik, K.; Dyda, M.; Wędzony, M. Changes in phenolic acid abundance involved in low temperature and Microdochium nivale (Samuels and Hallett) cross-tolerance in winter triticale (x Triticosecale Wittmack). Acta Physiol. Plant. 2019, 41, 38. [Google Scholar] [CrossRef] [Green Version]
- Dubas, E.; Golebiowska, G.; Zur, I.; Wedzony, M. Microdochium nivale (Fr., Samuels & Hallett): Cytological analysis of the infection process in triticale (×Triticosecale Wittm.). Acta Physiol. Plant. 2011, 33, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Żur, I.A.; Dubas, E.; Pociecha, E.; Dubert, F.; Kolasińska, I.; Płażek, A. Cytological analysis of infection process and the first defence responses induced in winter rye (Secale cereale L.) seedlings inoculated with Microdochium nivale. Phyiol. Mol. Plant Pathol. 2011, 76, 189–196. [Google Scholar] [CrossRef]
- Bertrand, A.; Castonguay, Y.; Azaiez, A.; Hsiang, T.; Dionne, J. Cold-induced responses in annual bluegrass genotypes with differential resistance to pink snow mold (Microdochium nivale). Plant Sci. 2011, 180, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gawronska, K.; Gołębiowska-Pikania, G. The effects of cold-hardening and Microdochium nivale infection on oxidative stress and antioxidative protection of the two contrasting genotypes of winter triticale. Eur. Food Res. Technol. 2016, 242, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Marzec-Schmidt, K.; Hura, K.; Płażek, A. Changes in antioxidants activity in grasses from complex Lolium-Festuca as a response to Microdochium nivale infection. Physiol. Mol. Plant Pathol. 2018, 104, 40–47. [Google Scholar] [CrossRef]
- Tronsmo, A.M. Predisposing effects of low temperature on resistance to winter stress factors in grasses. Acta Agric. Scand. 1984, 34, 210–220. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Chen, T.H.H. Effects of hardening and plant age on development of resistance to cottony snow mold (Coprinus psychromorbidus) in winter wheat under controlled conditions. Can. J. Bot. 1987, 65, 1152–1156. [Google Scholar] [CrossRef]
- Kuwabara, C.; Imai, R. Molecular Basis of Disease resistance acquired through cold acclimation in overwintering Plants. J. Plant Biol. 2009, 52, 19–26. [Google Scholar] [CrossRef]
- Tronsmo, A.M. Resistance to the rust fungus Puccinia poae-nemoralis in Poa pratensis induced by low-temperature hardening. Can. J. Bot. 1984, 62, 2891–2892. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Wang, Y.; Frick, M.; Puchalski, B.; Penniket, C.; Ouellet, T.; Robert, L.; Singh, J.; Laroche, A. Low temperature induced defence gene expression in winter wheat in relation to resistance to snow moulds and other wheat diseases. Plant Sci. 2011, 180, 99–110. [Google Scholar] [CrossRef]
- Pociecha, E.; Płażek, A.; Janowiak, F.; Waligórski, P.; Zwierzykowski, Z. Changes in abscisic acid, salicylic acid and phenylpropanoid concentrations during cold acclimation of androgenic forms of Festulolium (Festuca pratensis × Lolium multiflorum) in relation to resistance to pink snow mould (Microdochium nivale). Plant Breed. 2009, 128, 397–403. [Google Scholar] [CrossRef]
- Płażek, A.; Dubert, F.; Pociecha, E.; Janowiak, F.; Kolasińska, I.; Maciejewski, M. Resistance of winter rye (Secale cereale L.) to Microdochium nivale depends on soluble carbohydrate content but not on abscisic acid level. J. Phytopathol. 2011, 159, 751–758. [Google Scholar] [CrossRef]
- Christova, P.K.; Christov, N.K.; Imai, R. A Cold Inducible multidomain cystatin from winter wheat inhibits growth of the snow mold fungus, Microdochium nivale. Planta 2005, 223, 1207. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, C.; Takezawa, D.; Shimada, T.; Hamada, T.; Fujikawa, S.; Arakawa, K. Abscisic acid- and cold-induced thaumatin-like protein in winter wheat has an antifungal activity against snow mould, Microdochium nivale. Physiol. Plant. 2002, 115, 101–110. [Google Scholar] [CrossRef]
- Martin, L.; Fei, Z.; Giovannoni, J.; Rose, J. Catalyzing plant science research with RNA-Seq. Front. Plant Sci. 2013, 4, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogolev, Y.V.; Ahmar, S.; Akpinar, B.A.; Budak, H.; Kiryushkin, A.S.; Gorshkov, V.Y.; Hensel, G.; Demchenko, K.N.; Kovalchuk, I.; Mora-Poblete, F.; et al. Omics, epigenetics, and genome editing techniques for food and nutritional security. Plants 2021, 10, 1423. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, V.; Tsers, I. Plant susceptible responses: The underestimated side of plant–pathogen interactions. Biol. Rev. 2021, 97. [Google Scholar] [CrossRef] [PubMed]
- Kovi, M.R.; Abdelhalim, M.; Kunapareddy, A.; Ergon, Å.; Tronsmo, A.M.; Brurberg, M.B.; Hofgaard, I.S.; Asp, T.; Rognli, O.A. Global transcriptome changes in perennial ryegrass during early infection by pink snow mould. Sci. Rep. 2016, 6, 28702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, D.r.; Rezanoor, H.n.; Parry, D.w.; Nicholson, P. Evidence for differential host preference in Microdochium nivale var. majus and Microdochium nivale var. nivale. Plant Pathol. 2000, 49, 261–268. [Google Scholar] [CrossRef]
- Gorshkov, V.; Osipova, E.; Ponomareva, M.; Ponomarev, S.; Gogoleva, N.; Petrova, O.; Gogoleva, O.; Meshcherov, A.; Balkin, A.; Vetchinkina, E.; et al. Rye snow mold-associated Microdochium nivale strains inhabiting a common area: Variability in genetics, morphotype, extracellular enzymatic activities, and virulence. J. Fungi 2020, 6, 335. [Google Scholar] [CrossRef]
- Rabanus-Wallace, M.T.; Hackauf, B.; Mascher, M.; Lux, T.; Wicker, T.; Gundlach, H.; Baez, M.; Houben, A.; Mayer, K.F.X.; Guo, L.; et al. Chromosome-scale genome assembly provides insights into rye biology, evolution and agronomic potential. Nat. Genet. 2021, 53, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budínská, E.; et al. Dirigent proteins in plants: Modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, D.R. Respiration in plants interacting with pathogens, pests and parasitic plants. In Physiological Responses of Plants to Attack; John Wiley & Sons, Ltd.: Edinburgh, UK, 2015; pp. 88–113. ISBN 978-1-118-78305-4. [Google Scholar]
- Anwar, S.; Inselsbacher, E.; Grundler, F.M.W.; Hofmann, J. Arginine metabolism of Arabidopsis thaliana is modulated by Heterodera schachtii infection. Nematology 2015, 17, 1027–1043. [Google Scholar] [CrossRef]
- Tsers, I.; Gorshkov, V.; Gogoleva, N.; Parfirova, O.; Petrova, O.; Gogolev, Y. Plant soft rot development and regulation from the viewpoint of transcriptomic profiling. Plants 2020, 9, 1176. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the gramineae. Phytochemistry 1988, 27, 3349–3358. [Google Scholar] [CrossRef]
- Gao, Q.-M.; Venugopal, S.; Navarre, D.; Kachroo, A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 Proteins. Plant Physiol. 2011, 155, 464–476. [Google Scholar] [CrossRef] [Green Version]
- De Torres-Zabala, M.; Truman, W.; Bennett, M.H.; Lafforgue, G.; Mansfield, J.W.; Rodriguez Egea, P.; Bögre, L.; Grant, M. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007, 26, 1434–1443. [Google Scholar] [CrossRef]
- Yang, Y.; Sornaraj, P.; Borisjuk, N.; Kovalchuk, N.; Haefele, S.M. Transcriptional Network Involved in Drought Response and Adaptation in Cereals; IntechOpen: London, UK, 2016; ISBN 978-953-51-2250-0. [Google Scholar]
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.-J.; Gao, J.; Wang, P.; Duan, C.-G.; Zhu, X.; Zhu, J.-K. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci. Signal. 2014, 7, ra53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.S.; Jung, C.; Lee, S.; Min, K.; Lee, Y.-W.; Choi, Y.; Lee, J.S.; Song, J.T.; Kim, J.-K.; Choi, Y.D. AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J. 2013, 73, 483–495. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, C.-Z.; Ye, Q.; Wu, W.-H.; Chen, Y.-F. Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet. 2016, 12, e1005833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-L.; Shin, M.; Zou, X.; Huang, J.; Ho, T.D.; Shen, Q.J. A negative regulator encoded by a rice WRKY gene represses both abscisic acid and gibberellins signaling in aleurone cells. Plant Mol. Biol. 2009, 70, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; Tang, L.; Tong, X.; Wang, L.; Liu, L.; Huang, S.; Zhang, J. SAPK10-mediated phosphorylation on WRKY72 releases its suppression on jasmonic acid biosynthesis and bacterial blight resistance. iScience 2019, 16, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Tran, L.-S.P.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Gulli, M.; Maestri, E.; Hartings, H.; Raho, G.; Perrotta, C.; Devos, K.M.; Marmiroli, N. Isolation and characterization of abscisic acid inducible genes in barley seedlings and their responsiveness to environmental stress. Plant Physiol. Life Sci. Adv. 1995, 14, 89–96. [Google Scholar]
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaler, J.S.; Owen, B.; Higgins, V.J. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 2004, 135, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, R.; Shah, J. Salicylic acid in plant disease resistance. In Salicylic Acid A Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 335–370. ISBN 978-1-4020-5184-5. [Google Scholar]
- Zhang, N.; Zhou, S.; Yang, D.; Fan, Z. Revealing shared and distinct genes responding to JA and SA signaling in Arabidopsis by meta-analysis. Front. Plant Sci. 2020, 11, 908. [Google Scholar] [CrossRef]
- Mahmood, K.; Orabi, J.; Kristensen, P.S.; Sarup, P.; Jørgensen, L.N.; Jahoor, A. De novo transcriptome assembly, functional annotation, and expression profiling of rye (Secale cereale L.) hybrids inoculated with ergot (Claviceps purpurea). Sci. Rep. 2020, 10, 13475. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.-M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Väärtnõu, F.; Estrada, M.P.; Niinemets, Ü. Oak powdery mildew (Erysiphe alphitoides) induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014, 34, 1399–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, A.J.; Pelletier, G.; Tanguay, P.; Séguin, A. Transcriptome analysis of poplar during leaf spot infection with Sphaerulina spp. PLoS ONE 2015, 10, e0138162. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Tee, C.-S.; Jiang, Y.-L.; Jiang, X.-Y.; Venkatesh, P.N.; Sarojam, R.; Ye, J. A terpenoid phytoalexin plays a role in basal defense of Nicotiana benthamiana against Potato virus x. Sci. Rep. 2015, 5, 9682. [Google Scholar] [CrossRef] [Green Version]
- Pontin, M.; Bottini, R.; Burba, J.L.; Piccoli, P. Allium sativum produces terpenes with fungistatic properties in response to infection with Sclerotium cepivorum. Phytochemistry 2015, 115, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Pepori, A.L.; Michelozzi, M.; Santini, A.; Cencetti, G.; Bonello, P.; Gonthier, P.; Sebastiani, F.; Luchi, N. Comparative transcriptional and metabolic responses of Pinus pinea to a native and a non-native Heterobasidion species. Tree Physiol. 2019, 39, 31–44. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Y.; Luo, X.; Zhou, S. Comparative proteomic analysis provides insights into the complex responses to Pseudoperonospora cubensis infection of cucumber (Cucumis sativus L.). Sci. Rep. 2019, 9, 9433. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Watson, D.G. Terpenoid phytoalexins. Nat. Prod. Rep. 1991, 8, 367–389. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nicholson, R.L. Resistance of Maize to Anthracnose Colletotrichum graminicola: Changes in Host Phenols and Pigments. Phytopathology 1977, 67, 251–258. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Tian, J.; Yao, Y. Flavonoid accumulation plays an important role in the rust resistance of Malus plant leaves. Front. Plant Sci. 2017, 8, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, C.; Tsai, C.-J.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. salicylic acid activates poplar defense against the biotrophic rust fungus Melampsora larici-populina via increased biosynthesis of catechin and proanthocyanidins. New Phytol. 2019, 221, 960–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Skadhauge, B.; Thomsen, K.K.; Von Wettstein, D. The role of the barley testa layer and its flavonoid content in resistance to Fusarium infections. Hereditas 1997, 126, 147–160. [Google Scholar] [CrossRef]
- Chen, J.; Ullah, C.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Sclerotinia sclerotiorum circumvents flavonoid defenses by catabolizing flavonol glycosides and aglycones. Plant Physiol. 2019, 180, 1975–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, G.H.; Nicole, M.; Andary, C.; Martinez, C.; Bresson, E.; Boher, B.; Daniel, J.F.; Geiger, J.P. Flavonoids accumulate in cell walls, middle lamellae and callose-rich papillae during an incompatible interaction between Xanthomonas campestris pv. malvacearum and Cotton. Physiol. Mol. Plant Pathol. 1996, 49, 285–306. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Elder, T.; Kim, H.; Ralph, J. Lignin monomers from beyond the canonical monolignol biosynthetic pathway: Another brick in the wall. ACS Sustain. Chem. Eng. 2020, 8, 4997–5012. [Google Scholar] [CrossRef]
- Hou, S.; Jamieson, P.; He, P. The cloak, dagger, and shield: Proteases in plant-pathogen interactions. Biochem. J. 2018, 475, 2491–2509. [Google Scholar] [CrossRef]
- Godson, A.; van der Hoorn, R.A.L. The front line of defence: A meta-analysis of apoplastic proteases in plant immunity. J. Exp. Botany 2021, 72, 3381–3394. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Meier, B.M.; Croft, K.P.C.; Eiben, H.G. Lipoxygenase in plant disease. In Mechanisms of Plant Defense Responses; Fritig, B., Legrand, M., Eds.; Developments in Plant Pathology; Springer: Dordrecht, The Netherlands, 1993; pp. 211–220. ISBN 978-94-011-1737-1. [Google Scholar]
- Ongena, M.; Duby, F.; Rossignol, F.; Fauconnier, M.-L.; Dommes, J.; Thonart, P. Stimulation of the lipoxygenase pathway is associated with systemic resistance induced in bean by a nonpathogenic Pseudomonas strain. Mol. Plant-Microbe Interact. 2004, 17, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deboever, E.; Deleu, M.; Mongrand, S.; Lins, L.; Fauconnier, M.-L. Plant–pathogen interactions: Underestimated roles of phyto-oxylipins. Trends Plant Sci. 2019, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, T.; Kiriaki, M.; Ohgiya, S.; Fujiwara, M.; Kondo, H.; Nishimiya, Y.; Yumoto, I.; Tsuda, S. Antifreeze proteins from snow mold fungi. Can. J. Bot. 2003, 81, 1175–1181. [Google Scholar] [CrossRef]
- Istokovics, A.; Morita, N.; Izumi, K.; Hoshino, T.; Yumoto, I.; Sawada, M.T.; Ishizaki, K.; Okuyama, H. Neutral lipids, phospholipids, and a betaine lipid of the snow mold fungus Microdochium nivale. Can. J. Microbiol. 1998, 44, 1051–1059. [Google Scholar] [CrossRef]
- Okuyama, H. Effects of growth temperature on lipid and fatty acid compositions of the snow mold fungus. Microdochium nivale. Adv. Plant Lipid Res. 1998, 572, 598–601. [Google Scholar]
- Hoshino, T.; Ohgiya, S.; Shimanuki, T.; Ishizaki, K. Production of low temperature active lipase from the pink snow mold, Microdochium nivale (syn. Fusarium nivale). Biotechnol. Lett. 1996, 18, 509. [Google Scholar] [CrossRef]
- Song, W.; Ma, X.; Tan, H.; Zhou, J. Abscisic acid enhances resistance to Alternaria solani in tomato seedlings. Plant Physiol. Biochem. 2011, 49, 693–700. [Google Scholar] [CrossRef]
- Toueni, M.; Ben, C.; Le Ru, A.; Gentzbittel, L.; Rickauer, M. Quantitative resistance to Verticillium wilt in Medicago truncatula involves eradication of the fungus from roots and is associated with transcriptional responses related to innate immunity. Front. Plant Sci. 2016, 7, 1431. [Google Scholar] [CrossRef] [Green Version]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef] [Green Version]
- Ghorai, N.; Chakraborty, S.; Guchhait, S.; Saha, S.; Biswas, S. Estimation of total terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protoc. Exch. 2012. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A Comparative Study. Evid. Based Complement. Alternat. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Struchkov, P.; Beloborodov, V.; Kolkhir, V.; Voskoboynikova, I.; Savvateev, A. Comparison of spectrophotometric methods of total flavonoid assay based on complex formation with aluminum chloride as applied to multicomponent herbal drug angionorm. J. Pharm. Negat. Results 2018, 9, 1. [Google Scholar] [CrossRef]
- Akpinar, O.; Penner, M.H. Peptidase activity assays using protein substrates. Curr. Prot. Food Anal. Chem. 2002, 3, C2.2.1–C2.2.10. [Google Scholar] [CrossRef]
- Margesin, R.; Feller, G.; Hämmerle, M.; Stegner, U.; Schinner, F. A colorimetric method for the determination of lipase activity in soil. Biotechnol. Lett. 2002, 24, 27–33. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal rnas in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-Seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-Seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsers, I.; Meshcherov, A.; Gogoleva, O.; Petrova, O.; Gogoleva, N.; Ponomareva, M.; Gogolev, Y.; Korzun, V.; Gorshkov, V. Alterations in the Transcriptome of Rye Plants following the Microdochium nivale Infection: Identification of Resistance/Susceptibility-Related Reactions Based on RNA-Seq Analysis. Plants 2021, 10, 2723. https://doi.org/10.3390/plants10122723
Tsers I, Meshcherov A, Gogoleva O, Petrova O, Gogoleva N, Ponomareva M, Gogolev Y, Korzun V, Gorshkov V. Alterations in the Transcriptome of Rye Plants following the Microdochium nivale Infection: Identification of Resistance/Susceptibility-Related Reactions Based on RNA-Seq Analysis. Plants. 2021; 10(12):2723. https://doi.org/10.3390/plants10122723
Chicago/Turabian StyleTsers, Ivan, Azat Meshcherov, Olga Gogoleva, Olga Petrova, Natalia Gogoleva, Mira Ponomareva, Yuri Gogolev, Viktor Korzun, and Vladimir Gorshkov. 2021. "Alterations in the Transcriptome of Rye Plants following the Microdochium nivale Infection: Identification of Resistance/Susceptibility-Related Reactions Based on RNA-Seq Analysis" Plants 10, no. 12: 2723. https://doi.org/10.3390/plants10122723
APA StyleTsers, I., Meshcherov, A., Gogoleva, O., Petrova, O., Gogoleva, N., Ponomareva, M., Gogolev, Y., Korzun, V., & Gorshkov, V. (2021). Alterations in the Transcriptome of Rye Plants following the Microdochium nivale Infection: Identification of Resistance/Susceptibility-Related Reactions Based on RNA-Seq Analysis. Plants, 10(12), 2723. https://doi.org/10.3390/plants10122723