Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative Analysis of Phytochemical Compounds
2.2. Phenol and Flavonoid Content
2.3. Antioxidant Activity
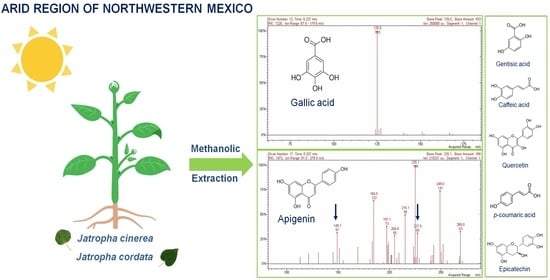
2.4. Identification of Phenolic Compounds by the Extract ESI-IT-MS-MS
2.5. HPLC-DAD Analyses of Methanolic Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of Sample
3.4. Extraction of Phenolic Compounds
3.5. Qualitative Analyses of Phytochemical Compounds
3.6. Determination of Total Phenolic Compounds
3.7. Determination of Total Flavonoids
3.8. Antioxidant Activity
3.8.1. DPPH Method Antioxidant Capacity
3.8.2. ABTS Method
3.9. Identification of Phenolic Compounds by ESI-IT-MS-MS
3.10. HPLC-DAD Analysis of Extracts
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, G.L. Classification of the Euphorbiaceae. Ann. Mo. Bot. Gard. 1994, 81, 3–32. [Google Scholar] [CrossRef]
- Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A.; Carter, S. World Checklist and Bibliography of Euphorbiaceae (with Pandaceae); Royal Botanic Gardens: Kew Richmond, UK, 2000. [Google Scholar]
- Steinmann, V.W. Diversidad y endemismo de la familia Euphorbiaceae en México. Acta Bot. Mex. 2002, 61–93. [Google Scholar] [CrossRef]
- Gübitz, G. Exploitation of the tropical oil seed plant Jatropha curcas L. Bioresour. Technol. 1999, 67, 73–82. [Google Scholar] [CrossRef]
- Sabandar, C.W.; Ahmat, N.; Jaafar, F.M.; Sahidin, I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef]
- Fresnedo-Ramírez, J.; Orozco–Ramírez, Q. Diversity and distribution of genus Jatropha in Mexico. Genet. Resour. Crop. Evol. 2012, 60, 1087–1104. [Google Scholar] [CrossRef]
- Córdova-Téllez, L.C.; Ramírez, E.B.; Colmenero, A.Z.; Lorca, J.A.R.; Vázquez, A.P.; Sánchez, O.M.S.; Herrera, J.M.; Sánchez, J.A.C. Diagnóstico y Plan Estratégico de Jatropha spp. En México; SNICS: Sinarefi, México, 2015. [Google Scholar]
- Schmid, R.; Turner, R.J.; Bowers, J.E.; Burgess, T.L. Sonoran Desert Plants: An Ecological Atlas; The University of Arizona Press: Tucson, AZ, USA, 2005. [Google Scholar]
- Dehgan, B.; Webster, G.L. Morphology and Infrageneric Relationships of the Genus Jatropha (Euphorbiaceae); University of California Press: Berkeley, CA, USA, 1979; Volume 74. [Google Scholar]
- Popham, A.R. Developmental anatomy of seedling of Jatropha cordata. Ohio J. Sci. 1947, 47, 1–20. [Google Scholar]
- Kane, C.W. Herbal Medicine of the American Southwest: A Guide to the Medicinal and Edible Plants of the Southwestern United States; Lincoln Town Press: Tucson, AZ, USA, 2006. [Google Scholar]
- Felger, R.S.; Johnson, M.B.; Wilson, M.F. The trees of Sonora, Mexico; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Felger, R.S.; Moser, M.B. Seri Indian pharmacopoeia. Econ. Bot. 1973, 28, 415–436. [Google Scholar] [CrossRef]
- Johnson, M.B. Jatropha (Euphorbiaceae) in Southwestern United States and Adjacent Northern Mexico; University of Arizona: Tucson, AZ, USA, 1998. [Google Scholar]
- Cavalcante, N.B.; Santos, A.D.D.C.; Almeida, J.R.G.D.S. The genus Jatropha (Euphorbiaceae): A review on secondary chemical metabolites and biological aspects. Chem. Interactions 2020, 318, 108976. [Google Scholar] [CrossRef]
- Srinivasan, N.; Palanisamy, K.; Mulpuri, S. Jatropha: Phytochemistry, Pharmacology, and Toxicology. In Jatropha, Challenges for a New Energy Crop; Springer Nature: Singapore, 2019; pp. 415–435. [Google Scholar]
- Rebecca, R.; Samuel, D.D.; Bello, Y.M.; Simeon, O.K. Qualitative Phytochemistry and Antibacterial Resistance Pattern of Leaves and Stem Bark Extracts of Jatropha curcas. Am. J. Microbiol. Res. 2016, 4, 143–146. [Google Scholar] [CrossRef]
- Oyama, M.O.; Malachi, O.I.; Oladejo, A.A. Phytochemical Screening and Antimicrobial Activity of Leaf Extract of Jatropha curcas. J. Adv. Med. Pharm. Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Ascacio-Valdés, J.A.; Cruz, K.; Reyes-Luna, C.; Rodríguez, R.; Aguilar, C.N. Chapter 5-Extraction of Bioactive Phenolic Compounds by Alternative Technologies. In Handbook of Food Bioengineering, Ingredients Extraction by Physicochemical Methods in Food; Academic Press: New York, NY, USA, 2017; pp. 229–252. [Google Scholar] [CrossRef]
- Dias, W.; Junior, E.D.V.; Oliveira, M.D.D.A.D.; Barbosa, Y.; Silva, J.D.N.; Júnior, J.D.C.; De Almeida, P.; Martins, F. Cytogenotoxic effect, phytochemical screening and antioxidant potential of Jatropha mollissima (Pohl) Baill leaves. S. Afr. J. Bot. 2019, 123, 30–35. [Google Scholar] [CrossRef]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Ismail, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namuli, A.; Abdullah, N.; Sieo, C.; Zuhainis, S.; Oskoueian, E. Phytochemical compounds and antibacterial activity of Jatropha curcas Linn. extracts. J. Med. Plants Res. 2011, 5, 3982–3990. [Google Scholar]
- El-Baz, F.K.; Aly, H.F.; Abd-Alla, H.I.; Saad, S.A. Bioactive flavonoid glycosides and antidiabetic activity of Jatropha curcas on streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 143–156. [Google Scholar]
- Jain, S.; Choudhary, G.; Jaina, D. In vitro free radical scavenging activity of Jatropha gossgypifolia Linn. containing phenolic compounds. J. Med. Plants Res. 2013, 7, 1424–1428. [Google Scholar] [CrossRef]
- Rampadarath, S.; Puchooa, D.; Mala Ranghoo-Sanmukhiya, V. A comparison of polyphenolic content, antioxidant activity and insecticidal properties of Jatropha species and wild Ricinus communis L. found in Mauritius. Asian Pac. J. Trop. Med. 2014, 7, S384–S390. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.B.E.H.; Bahri, R.; Chaouachi, M.; Boussaïd, M.; Harzallah-Skhiri, F. Phenolic content, antioxidant and allelopathic activities of various extracts of Thymus numidicus Poir. organs. Ind. Crop. Prod. 2014, 62, 188–195. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Banjarnahor, S.D.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2015, 23, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.B.L.; Lim, Y.Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F. Spectroscopic Analyses: Developments and Applications; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. Jatropha gossypiifolia L. and its biologically active metabolites: A mini review. J. Ethnopharmacol. 2019, 234, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.D.S.; Leal, G.C.; Molin, T.R.D.; Faccin, H.; Gobo, L.A.; Da Silveira, G.D.; Souza, M.T.D.S.; Lameira, O.A.; De Carvalho, L.M.; Viana, C. Determination of phenolic and triterpenic compounds in Jatropha gossypiifolia L by Ultra-high performance liquid chromatography-tandem mass spectrometric (UHPLC-MS/MS). Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Khallouki, F.; Voggel, J.; Breuer, A.; Klika, K.D.; Ulrich, C.M.; Owen, R. Comparison of the major polyphenols in mature Argan fruits from two regions of Morocco. Food Chem. 2017, 221, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.D.A.; Stringheta, P.C.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Flavonols and ellagic acid derivatives in peels of different species of jabuticaba (Plinia spp.) identified by HPLC-DAD-ESI/MSn. Food Chem. 2018, 252, 61–71. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts-review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, A.; Alarcon-Aguilar, F.; Almanza-Perez, J.; Nieto-Yañez, O.; Olivares-Sanchez, J.; Duran-Diaz, A.; Rodriguez-Monroy, M.; Canales-Martinez, M. Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. J. Ethnopharmacol. 2017, 204, 1–7. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Dias, A.L.D.S.; Rozet, E.; Larondelle, Y.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. Development and validation of an UHPLC-LTQ-Orbitrap MS method for non-anthocyanin flavonoids quantification in Euterpe oleracea juice. Anal. Bioanal. Chem. 2013, 405, 9235–9249. [Google Scholar] [CrossRef]
- Papalia, T.; Barreca, D.; Panuccio, M.R. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, H.A.S.; Ribeiro, L.F.; Pirovani, C.P.; Gramacho, K.P.; Micheli, F. Activity of polygalacturonases from Moniliophthora perniciosa depends on fungus culture conditions and is enhanced by Theobroma cacao extracts. Physiol. Mol. Plant Pathol. 2013, 83, 40–50. [Google Scholar] [CrossRef]
- Haddock, E.A.; Gupta, R.K.; Al-Shafi, S.M.K.; Haslam, E.; Magnolato, D. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part Introduction. Naturally occurring galloyl esters. J. Chem. Soc. Perkin Trans. 1982, 1, 2515–2524. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M. Variation in phenolic compounds, α-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L.) during phenological growth stages. Physiol. Mol. Biol. Plants 2020, 26, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. Antioxidant activity and free radical scavenging reactions of gentisic acid: In-vitro and pulse radiolysis studies. Free Radic. Res. 2011, 46, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2019, 34, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Darabi, P.; Khazali, H.; Natanzi, M.M. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: Via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecol. Endocrinol. 2019, 36, 582–587. [Google Scholar] [CrossRef]
- Molina-Quijada, D.M.; Medina-Juárez, L.A.; González-Aguilar, G.A.; Robles-Sánchez, R.M.; Gamez-Meza, N. Compuestos fenólicos y actividad antioxidante de cáscara de uva (Vitis viniferaL.) de mesa cultivada en el noroeste de México Phenolic compounds and antioxidant activity of table grape (Vitis viniferaL.) skin from northwest Mexico. CyTA J. Food 2010, 8, 57–63. [Google Scholar] [CrossRef]
- Janaki, A.; Kaleena, P.K.; Elumalai, D.; Velu, K.; Babu, M.; Ravi, S.; Hemavathi, M.; Arulvasu, C. Qualitative and quantitative phytochemical analysis: In vitro studies of antioxidant and anticancer activity of Bauhinia tomentosa (L) leaf extracts. J. Pharmacogn. Phytochem. 2018, 7, 2403–2410. [Google Scholar]
- Nayak, A.; Satapathi, K.; Sahoo, S. Comparative Studies on the Phytochemistry, Antimicrobial and Antioxidant Activities of Jatropha Species (J. curcas L. and J. gossypifolia L.) of Odisha. J. Pharmacogn. Phytochem. Res. 2016, 8, 1614–1624. [Google Scholar]
- Hassan, H.A.; Attia, E.Z.; Desoukey, S.Y.; Mohamed, K.M.; Kamel, M. Quantitative Analysis of Total Phenolic and Total Flavonoid Constituents of some Ficus species. J. Adv. Biomed. Pharm. Sci. 2019, 2, 38–40. [Google Scholar] [CrossRef] [Green Version]
- Elosaily, A.H.; Mahrous, E.A.; Salama, A.M.; El Zalabani, S.M. Proximate composition, phenolic content and antioxidant potential of the leaves of four Jatropha species. Int. J. Res. Pharm. Sci. 2019, 10, 419–424. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Campos, M.V.; Loyola, E.; Dueñas, M.; Santos, C. ACTIVIDAD ANTIOXIDANTE DE METABOLITOS DE FLAVONOIDES ORIGINADOS POR LA MICROFLORA DEL INTESTINO HUMANO. Rev. Soc. Química Perú 2016, 82, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Cantos, E.; García-Viguera, C.; De Pascual-Teresa, S.; Tomás-Barberán, F.A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef]



| Phytochemicals | J. cinerea | J. cordata | ||||||
|---|---|---|---|---|---|---|---|---|
| H | EA | E | M | H | EA | E | M | |
| Flavonoids | − | − | + | + | − | − | + | + |
| Phenols | − | − | + | + | − | − | + | + |
| Coumarins | − | + | + | + | − | − | − | − |
| Saponins | − | − | − | − | − | − | − | − |
| Sterols | + | − | − | − | + | − | − | − |
| Tannins | − | − | + | + | − | − | + | + |
| Alkaloids | − | − | − | − | − | − | − | − |
| Quinones | − | − | − | − | − | − | − | − |
| Glycosides | − | − | − | − | − | − | − | − |
| J. cinerea | J. cordata | |||
|---|---|---|---|---|
| Leaves | Stems | Leaves | Stems | |
| Total phenols (mg GAE/g) | 82.65 ± 1.65 * | 62.02 ± 2.80 * | 215.63 ± 3.62 * | 153.39 ± 2.91 * |
| Total flavonoids (mg QE/g) | 11.30 ± 0.13 * | 3.27 ± 0.11 * | 18.55 ± 0.23 * | 7.41 ± 0.14 * |
| ABTS IC50 (mg/mL) | 23.03 ± 2.31 * | 15.92 ± 0.01 * | 7.59 ± 1.10 | 7.18 ± 0.99 |
| DPPH IC50 (mg/mL) | 14.03 ± 2.40 | 12.50 ± 1.30 | 2.72 ± 0.46 * | 3.96 ± 0.39 * |
| Compounds | Precursor Ion | Fragment Ions | J. cinerea | J. cordata | |||
|---|---|---|---|---|---|---|---|
| [M-H]-m/z | ESI-MSN (m/z) | Leaves | Stems | Leaves | Stems | ||
| 1 | 3,4-Dihydroxy-benzoic acid [33] | 153 | 109, 153, 108 | * | * | * | * |
| 2 | 4-Hydroxy-benzoic acid ° | 137 | 92, 93, 136, 137 | * | * | * | * |
| 3 | Caffeic acid ° | 179 | 135 | * | * | * | * |
| 4 | Ellagic acid [34] | 301 | 300.9, 229, 257 | * | * | * | * |
| 5 | Gallic acid ° | 169 | 125 | * | * | * | * |
| 6 | Gentisic acid ° | 153 | 109 | * | * | * | * |
| 7 | p-coumaric acid ° | 163 | 119 | * | * | * | * |
| 8 | Syringic acid ° | 197 | 182, 153 | ND | * | ND | ND |
| 9 | Sinapic acid ° | 223 | 164, 179 | ND | ND | ND | * |
| 10 | Tannic acid [35] | 1700 | 1700 | * | * | * | * |
| 11 | Vanillic acid ° | 167 | 157, 123, 108 | ND | * | ND | * |
| 12 | Apigenin ° | 269 | 227, 150, 117 | * | * | * | * |
| 13 | Catechol [36] | 109 | 108, 91 | * | * | * | * |
| 14 | Epicatechin ° | 289 | 245, 205, 179 | ND | * | ND | * |
| 15 | Apigenin-7-O-Rutinoside [37] | 577 | 269, 577 | * | ND | ND | ND |
| 16 | Rutin hydrate [38] | 609 | 301, 151 | ND | ND | * | * |
| 17 | Isovitexin [39] | 431 | 311, 341, 413 | * | * | * | * |
| 18 | Pyrogalol [39] | 125 | 124, 125 | ND | ND | ND | * |
| 19 | Quercetin ° | 301 | 151, 179 | ND | * | * | * |
| 20 | Rhoifolin [39] | 577 | 577.1, 269, 268 | * | ND | ND | ND |
| 21 | Rutin ° | 609 | 301, 179,151 | ND | * | * | * |
| 22 | Vitexin [39] | 431 | 311,341 | * | ND | * | * |
| Compounds | J. cinerea | J. cordata | ||
|---|---|---|---|---|
| Leaves | Stems | Leaves | Stems | |
| 4-Hydroxybenzoic acid | 60.34 ± 0.50 * | 35.97 ± 0.10 * | 75.74 ± 0.89 * | 22.09 ± 0.62 * |
| Caffeic acid | 61.27 ± 0.10 * | 22.19 ± 0.10 * | 58.75 ± 0.31 * | 50.49 ± 0.31 * |
| Gallic acid | 261.68 ± 0.40 * | 113.77 ± 0.60 * | 284.45 ± 1.27 * | 215.31 ± 1.19 * |
| Gentisic acid | 634.55 ± 1.80 * | 26.91 ± 0.40 * | 864.62 ± 5.24 * | 14.6 ± 0.21 * |
| p-coumaric acid | 175.27 ± 0.40 * | 62.98 ± 0.50 * | 123.91 ± 0.13 * | ND |
| Sinapic acid | ND | ND | ND | 12.44 ± 0.12 * |
| Syringic acid | ND | 39.15 ± 0.60 * | ND | ND |
| Vanillic acid | ND | 38.48 ± 0.10 * | ND | 40.81 ± 0.80 * |
| Apigenin | 148.06 ± 1.30 * | 94.18 ± 1.80 * | 343.54 ± 2.96 * | 87.61 ± 1.46 * |
| Epicatechin | ND | 230.05 ± 4.60 * | ND | 90.16 ± 6.79 * |
| Quercetin | ND | 198.52 ± 0.20 * | 238.12 ± 1.63 * | 573.98 ± 2.11 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Ruiz, Y.C.; Hayano-Kanashiro, C.; Gámez-Meza, N.; Medina-Juárez, L.A. Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg. Plants 2021, 10, 212. https://doi.org/10.3390/plants10020212
Vega-Ruiz YC, Hayano-Kanashiro C, Gámez-Meza N, Medina-Juárez LA. Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg. Plants. 2021; 10(2):212. https://doi.org/10.3390/plants10020212
Chicago/Turabian StyleVega-Ruiz, Yeimi Cecilia, Corina Hayano-Kanashiro, Nohemí Gámez-Meza, and Luis Angel Medina-Juárez. 2021. "Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg" Plants 10, no. 2: 212. https://doi.org/10.3390/plants10020212
APA StyleVega-Ruiz, Y. C., Hayano-Kanashiro, C., Gámez-Meza, N., & Medina-Juárez, L. A. (2021). Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg. Plants, 10(2), 212. https://doi.org/10.3390/plants10020212