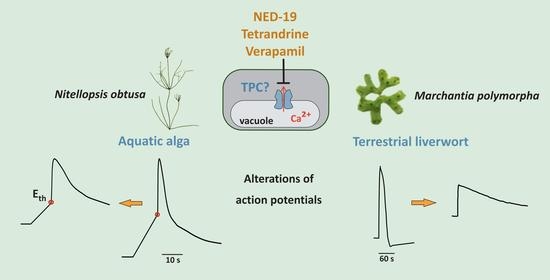
Impact of Mammalian Two-Pore Channel Inhibitors on Long-Distance Electrical Signals in the Characean Macroalga Nitellopsis obtusa and the Early Terrestrial Liverwort Marchantia polymorpha
Abstract
:1. Introduction
2. Results
2.1. Action Potentials in Nitellopsis obtusa Cells
2.2. Action Potential Conduction Velocity Along an Internodal N. obtusa Cell
2.3. Patch Clamp Measurements on N. obtusa Cytoplasmic Droplet Membrane
2.4. Action Potentials in Marchantia polymorpha Cells
2.5. Patch Clamp Measurements on Vacuoles Isolated from Marchantia polymorpha Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and Tested Drugs
4.2. Measurements in Nitellopsis obtusa
4.2.1. Intracellular Microelectrode Recordings
4.2.2. Investigation of AP Conduction Velocity
4.2.3. Patch Clamping of Cytoplasmic Droplets
4.2.4. Data Analysis
4.3. Measurements in Marchantia polymorpha
4.3.1. Intracellular Microelectrode Recordings
4.3.2. Stimuli
4.3.3. Data Analysis
4.3.4. Patch Clamp Recordings and Analysis of Marchantia polymorpha Vacuolar Channels
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- She, J.; Guo, J.; Chen, Q.; Zeng, W.; Jiang, Y.; Bai, X. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 2018, 556, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Penny, C.J.; Vassileva, K.; Jha, A.; Yuan, Y.; Chee, X.; Yates, E.; Mazzon, M.; Kilpatrick, B.S.; Muallem, S.; March, M.; et al. Mining Ebola virus entry inhibitors identifies approved drugs as two-pore channel blockers. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1151–1161. [Google Scholar] [CrossRef]
- Patel, S.; Kilpatrick, B.S. Two-pore channels and disease. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1678–1686. [Google Scholar] [CrossRef]
- Hedrich, R.; Neher, E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 1987, 329, 833–835. [Google Scholar] [CrossRef]
- Furuichi, T.; Cunningham, K.W.; Muto, S. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001, 42, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Peiter, E.; Maathuis, F.J.M.; Mills, L.N.; Knight, H.; Pelloux, J.; Hetherington, A.M.; Sanders, D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 2005, 434, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Sakurai, S.; Miyao, A.; Hirochika, H.; Kuchitsu, K. Identification of a putative voltage-gated Ca2+-permeable channel (OsTPC1) involved in Ca2+ influx and regulation of growth and development in rice. Plant Cell Physiol. 2004, 45, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Hamada, H.; Kurusu, T.; Okuma, E.; Nokajima, H.; Kiyoduka, M.; Koyano, T.; Sugiyama, Y.; Okada, K.; Koga, J.; Saji, H.; et al. Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured rice cells. J. Biol. Chem. 2012, 287, 9931–9939. [Google Scholar] [CrossRef] [Green Version]
- Dadacz-Narloch, B.; Kimura, S.; Kurusu, T.; Farmer, E.E.; Becker, D.; Kuchitsu, K.; Hedrich, R. On the cellular site of two-pore channel TPC1 action 1 in the Poaceae. New Phytol. 2013, 200, 663–674. [Google Scholar] [CrossRef]
- Kadota, Y.; Furuichi, T.; Ogasawara, Y.; Goh, T.; Higashi, K.; Muto, S.; Kuchitsu, K. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem. Biophys. Res. Comm. 2004, 317, 823–830. [Google Scholar] [CrossRef]
- Kurusu, T.; Yagala, T.; Miyao, A.; Hirochika, H.; Kuchitsu, K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005, 42, 798–809. [Google Scholar] [CrossRef]
- Kadota, Y.; Furuichi, T.; Sano, T.; Kaya, H.; Murakami, Y.; Gunji, W.; Muto, S.; Hasezawa, S.; Kuchitsu, K. Cell cycle-dependent regulation of oxidative stress responses and Ca2+ permeable channels NtTPC1A/B in tobacco BY-2 cells. Biochem. Biophys. Res. Comm. 2005, 336, 1259–1267. [Google Scholar] [CrossRef]
- Guo, J.T.; Zeng, W.Z.; Chen, Q.F.; Lee, C.; Chen, L.P.; Yang, Y.; Cang, C.L.; Ren, D.J.; Jiang, Y.X. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Pottosin, I.I.; Schӧnknecht, G. Vacuolar calcium channels. J. Exp. Bot. 2007, 58, 1559–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrich, R.; Marten, I. TPC1-SV channels gain shape. Mol. Plant 2011, 4, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Mueller, T.D.; Becker, D.; Marten, I. Structure and function of TPC1 vacuole SV channel gains shape. Mol. Plant 2018, 11, 764–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyhl, D.; Hoertensteiner, S.; Martinoia, E.; Farmer, E.E.; Fromm, J.; Marten, I.; Hedrich, R. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 2009, 58, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Dadacz-Narloch, B.; Beyhl, D.; Larisch, C.; Lopez-Sanjurjo, E.J.; Reski, R.; Kuchitsu, K.; Muller, T.D.; Becker, D.; Schӧnknecht, G.; Hedrich, R. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 2011, 23, 2696–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, J.M.; Schroeder, J.I. Calcium-Activated K+ Channels and Calcium-Induced Calcium Release by Slow Vacuolar Ion Channels in Guard Cell Vacuoles Implicated in the Control of Stomatal Closure. Plant Cell 1994, 6, 669–683. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Tikhonova, L.I.; Hedrich, R.; Schönknecht, G. Slowly activating vacuolar channels cannot mediate Ca2+-induced Ca2+ release. Plant J. 1997, 12, 1387–1398. [Google Scholar] [CrossRef]
- Ranf, S.; Wünnenberg, P.; Lee, J.; Becker, D.; Dunkel, M.; Hedrich, R.; Scheel, D.; Dietrich, P. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 2008, 53, 287–299. [Google Scholar] [CrossRef]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiep, V.; Vadassery, J.; Lattke, J.; Maass, J.P.; Boland, W.; Peiter, E.; Mithöfer, A. Systemic cytosolic Ca2+ elevations activated upon wounding and herbivory in Arabidopsis. New Phytol. 2015, 207, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, K. Evolution of land plants: Insights from molecular studies on basal lineages. Biosci. Biotechnol. Biochem. 2017, 81, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupisz, K.; Dziubinska, H.; Trebacz, K. Generation of action potential-type changes in response to darkening and illumination as indication of the plasma membrane proton pump status in Marchantia polymorpha. Acta Physiol Plant 2017, 39, 82. [Google Scholar] [CrossRef] [Green Version]
- Pupkis, V.; Buisas, R.; Lapeikaitė, I.; Kisnierienė, V. Employing plant cells of Nitellopsis obtusa for biophysical education. Biophysicist 2020. [Google Scholar] [CrossRef]
- Trębacz, K.; Schӧnknecht, G. Simple method to isolate vacuoles and protoplasts for patch-clamp experiments. Protoplasma 2000, 213, 39–45. [Google Scholar] [CrossRef]
- Koselski, M.; Trebacz, K.; Dziubinska, H. Cation-permeable vacuolar ion channels in the moss Physcomitrella patens: A patch-clamp study. Planta 2013, 238, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Lunevsky, V.Z.; Zherelova, O.M.; Vostrikov, I.Y.; Berestovsky, G.N. Excitation of Characeae cell membranes as a result of activation of calcium and chloride channels. J. Membr. Biol. 1983, 72, 43–58. [Google Scholar] [CrossRef]
- Trębacz, K.; Dziubińska, H.; Król, E. Electrical signals in long-distance communication in plants. In Communication in Plants: Neuronal Aspects of Plant Life; Baluska, F., Mancuso, S., Volkmann, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 277–290. [Google Scholar]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Shimmen, T.; Nishikawa, S.I. Studies on the tonoplast action potential of Nitella flexilis. J. Membr. Biol. 1988, 101, 133–140. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sakayama, H.; De Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Rensing, S.A. The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 2018, 174, 448–464. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304. [Google Scholar] [CrossRef]
- Król, E.; Trębacz, K. Calcium-dependent voltage transients evoked by illumination in the liverwort Conocephalum conicum. Plant Cell Physiol. 1999, 40, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Trębacz, K.; Simonis, W.; Schönknecht, G. Effects of anion channel inhibitors on light-induced potential changes in the liverwort Conocephalum conicum. Plant Cell Physiol. 1997, 38, 550–557. [Google Scholar] [CrossRef]
- Król, E.; Dziubińska, H.; Trębacz, K. Low-temperature-induced transmembrane potential changes in mesophyll cells of Arabidopsis thaliana, Helianthus annuus and Vicia faba. Physiol. Plant. 2004, 120, 265–270. [Google Scholar] [CrossRef]
- Wacke, M.; Thiel, G.; Hütt, M.T. Ca2+ dynamics during membrane excitation of green alga Chara: Model simulations and experimental data. J. Membr. Biol. 2003, 191, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Beilby, M.J. Action potential in charophytes. Int. Rev. Cytol. 2007, 257, 43–82. [Google Scholar]
- Kisnieriene, V.; Lapeikaite, I.; Sevriukova, O.; Ruksenas, O. The effects of Ni2+ on electrical signaling of Nitellopsis obtusa cells. J. Plant Res. 2016, 129, 551–558. [Google Scholar] [CrossRef]
- Lapeikaite, I.; Pupkis, V.; Neniskis, V.; Ruksenas, O.; Kisnieriene, V. Glutamate and NMDA affect cell excitability and action potential dynamics of single cell of macrophyte Nitellopsis obtusa. Funct. Plant Biol. 2020, 47, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, I.; Ohkawa, T.A.; Nagai, R.; Kishimoto, U. Role of calcium ion in the excitability and electrogenic pump activity of the Chara corallina membrane: I. Effects of La3+, verapamil, EGTA, W-7, and TFP on the action potential. J. Membr. Biol. 1987, 96, 65–73. [Google Scholar] [CrossRef]
- Kisnieriene, V.; Lapeikaite, I.; Pupkis, V.; Beilby, M.J. Modeling the action potential in Characeae Nitellopsis obtusa: Effect of saline stress. Front. Plant Sci. 2019, 10, 82. [Google Scholar] [CrossRef]
- Boccaccio, A.; Scholz-Starke, J.; Hamamoto, S.; Larisch, N.; Festa, M.; Gutla, P.V.; Costa, A.; Dietrich, P.; Uozumi, N.; Carpaneto, A. The phosphoinositide PI(3,5)P2 mediates activation of mammalian but not plant TPC proteins: Functional expression of endolysosomal channels in yeast and plant cells. Cell. Mol. Life Sci. 2014, 71, 4275–4283. [Google Scholar] [CrossRef]
- Trębacz, K.; Simonis, W.; Schönknecht, G. Cytoplasmic Ca2+, K+, Cl−, and NO3− activities in the liverwort Conocephalum conicum L. at rest and during action potentials. Plant Physiol. 1994, 106, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Jaślan, D.; Dreyer, I.; Lu, J.; O’Malley, R.; Dindas, J.; Marten, I.; Hedrich, R. Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat. Commun. 2019, 10, 2659. [Google Scholar] [CrossRef] [Green Version]
- Laver, D.R. Divalent cation block and competition between divalent and monovalent cations in the large-conductance K+ channel from Chara australis. J. Gen. Physiol. 1992, 100, 269–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lühring, H. Recording of single K+ channels in the membrane of cytoplasmic drop of Chara australis. Protoplasma 1986, 133, 19–28. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Andjus, P.R. Depolarization-activated K+ channel in Chara droplets. Plant Physiol. 1994, 106, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuhara, M.; Mimura, T.; Tazawa, M. Patch-clamp study on ion channels in the tonoplast of Nitellopsis obtusa. Plant Cell Physiol. 1991, 32, 179–184. [Google Scholar] [CrossRef]
- Kisnierienė, V.; Lapeikaitė, I.; Pupkis, V. Electrical signalling in Nitellopsis obtusa: Potential biomarkers of biologically active compounds. Funct. Plant Biol. 2017, 45, 132–142. [Google Scholar] [CrossRef]
- Lapeikaite, I.; Dragunaite, U.; Pupkis, V.; Ruksenas, O.; Kisnieriene, V. Asparagine alters action potential parameters in single plant cell. Protoplasma 2019, 256, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, C.; Sachs, F. Solving ion channel kinetics with the QuB software. Biophys. Rev. Lett. 2013, 8, 191–211. [Google Scholar] [CrossRef]
- Koselski, M.; Trebacz, K.; Dziubinska, H. Vacuolar ion channels in the liverwort Marchantia polymorpha: Influence of ion channel inhibitors. Planta 2017, 245, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Bertl, A.; Blumwald, E.; Coronado, R.; Eisenberg, R.; Findlay, G.; Gradmann, D.; Hille, B.; Kohler, K.; Kolb, H.A.; MacRobbie, E.; et al. Electrical measurements on endomembranes. Science 1992, 258, 873–874. [Google Scholar] [CrossRef] [PubMed]





| APW | Verapamil 300 µM | APW | NED-19 75 µM | APW | Tetrandrine 100 µM | |
|---|---|---|---|---|---|---|
| RP (mV) | −210 ± 11 (n = 7) | −140 ± 62 (n = 7)* | 201 ± 15 (n = 7) | −155 ± 33 (n = 7)* | −203 ± 21 (n = 6) | −165 ± 44 (n = 6)* |
| Eth (mV) | −101 ± 12 (n = 7) | −73 ± 35 (n = 7)* | −108 ± 9 (n = 7) | −81 ± 20 (n = 7)* | −78 ± 18 (n = 6) | −80 ± 17 (n = 6) |
| Vmax (mV) | 36 ± 14 (n = 7) | 37 ± 24 (n = 7) | 35 ± 10 (n = 7) | 27 ± 25 (n = 7) | 30 ± 4 (n = 6) | 32 ± 6 (n = 6) |
| Ath (mV) | 137 ± 21 (n = 7) | 110 ± 52 (n = 7) | 143 ± 10 (n = 7) | 107 ± 31 (n = 7)* | 107 ± 18 (n = 6) | 112 ± 18 (n = 6) |
| ARP (mV) | 254 ± 14 (n = 7) | 178 ± 79 (n = 7)* | 231 ± 17 (n = 7) | 181 ± 33 (n = 7)* | 233 ± 21 (n = 6) | 197 ± 42 (n = 6) |
| tdep (s) | 0.8 ± 0.1 (n = 7) | 1.6 ± 0.7 (n = 7)* | 0.9 ± 0.1 (n = 7) | 1 ± 0.2 (n = 7) | 1.3 ± 0.2 (n = 6) | 1.9 ± 0.3 (n = 6)* |
| trep (s) | 2.6 ± 0.7 (n = 7) | 8 ± 8.6 (n = 7)* | 3.5 ± 1.2 (n = 7) | 6 ± 3.4 (n = 7) | 2.6 ± 0.8 (n = 6) | 8.2 ± 3.9 (n = 6)* |
| t1/2 (s) | 3.5 ± 0.8 (n = 7) | 9.6 ± 9.2 (n = 7)* | 4.4 ± 1.2 (n = 7) | 6.9 ± 3.4 (n = 7)* | 3.9 ± 0.9 (n = 6) | 10.1 ± 4 (n = 6)* |
| Standard | 20 µM Verapamil | 20 µM Tetrandrine | 75 µM NED-19 (short) | 75 µM NED-19 (long) | |
|---|---|---|---|---|---|
| RP (mV) | −187± 12 (n = 16) | −163 ± 10 (n = 10)* | −130 ± 12 (n = 8)* | −172 ± 17 (n = 8)* | −165 ± 21 (n = 6)* |
| Vmax (mV) | −47 ± 23 (n = 16) | −98 ± 34 (n=6)* | −58 ± 18 (n=8) | −46 ± 15 (n = 8) | −46 ± 13 (n = 6) |
| ARP (mV) | 139 ± 19 (n = 16) | 70 ± 33 (n = 6)* | 72 ± 13 (n = 8)* | 126 ± 9 (n = 8)* | 119 ± 11 (n = 6)* |
| tdep (s) | 4.8 ± 1.6 (n = 16) | 5.5 ± 1.7 (n = 6) | 5.9 ± 1.1 (n = 7) | 5.9 ± 1.6 (n = 8) | 6.1 ± 1.7 (n = 6) |
| trep (s) | 19.9 ± 6.5 (n = 16) | 13.4 ± 5.7 (n = 6) | 169.8 ± 76.2 (n = 7)* | 30.7 ± 13.0 (n = 8)* | 184.3 ± 46.2 (n = 6)* |
| t1/2 (s) | 24.7 ± 7.3 (n = 16) | 18.9 ±6.9 (n = 6) | 184.5 ± 74.8 (n = 8)* | 36.6 ± 12.1 (n = 8)* | 190.4 ± 45.4 (n = 6)* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koselski, M.; Pupkis, V.; Hashimoto, K.; Lapeikaite, I.; Hanaka, A.; Wasko, P.; Plukaite, E.; Kuchitsu, K.; Kisnieriene, V.; Trebacz, K. Impact of Mammalian Two-Pore Channel Inhibitors on Long-Distance Electrical Signals in the Characean Macroalga Nitellopsis obtusa and the Early Terrestrial Liverwort Marchantia polymorpha. Plants 2021, 10, 647. https://doi.org/10.3390/plants10040647
Koselski M, Pupkis V, Hashimoto K, Lapeikaite I, Hanaka A, Wasko P, Plukaite E, Kuchitsu K, Kisnieriene V, Trebacz K. Impact of Mammalian Two-Pore Channel Inhibitors on Long-Distance Electrical Signals in the Characean Macroalga Nitellopsis obtusa and the Early Terrestrial Liverwort Marchantia polymorpha. Plants. 2021; 10(4):647. https://doi.org/10.3390/plants10040647
Chicago/Turabian StyleKoselski, Mateusz, Vilmantas Pupkis, Kenji Hashimoto, Indre Lapeikaite, Agnieszka Hanaka, Piotr Wasko, Egle Plukaite, Kazuyuki Kuchitsu, Vilma Kisnieriene, and Kazimierz Trebacz. 2021. "Impact of Mammalian Two-Pore Channel Inhibitors on Long-Distance Electrical Signals in the Characean Macroalga Nitellopsis obtusa and the Early Terrestrial Liverwort Marchantia polymorpha" Plants 10, no. 4: 647. https://doi.org/10.3390/plants10040647
APA StyleKoselski, M., Pupkis, V., Hashimoto, K., Lapeikaite, I., Hanaka, A., Wasko, P., Plukaite, E., Kuchitsu, K., Kisnieriene, V., & Trebacz, K. (2021). Impact of Mammalian Two-Pore Channel Inhibitors on Long-Distance Electrical Signals in the Characean Macroalga Nitellopsis obtusa and the Early Terrestrial Liverwort Marchantia polymorpha. Plants, 10(4), 647. https://doi.org/10.3390/plants10040647