Opposite Growth Responses of Alexandrium minutum and Alexandrium catenella to Photoperiods and Temperatures
Abstract
:1. Introduction
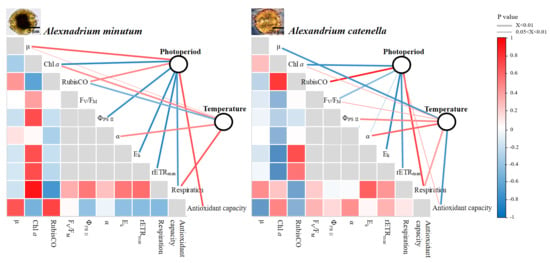
2. Results
2.1. Growth Rate
2.2. Cell Compositions
2.3. Chlorophyll Fluorescence
2.4. Dark Respiration and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Culture Protocol
4.2. Experimental Design
4.2.1. Growth Rate
4.2.2. Maximal PSII Quantum Yield
4.2.3. Dark Respiration
4.2.4. Anti-Oxidant Activity
4.2.5. Cellular Composition
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischetti, M. Deep heat threatens marine life. Sci. Am. 2013, 308, 92. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Thomas, M.K.; Kremer, C.T.; Klausmeier, C.A.; Litchman, E. A global pattern of thermal adaptation in marine phytoplankton. Science 2012, 338, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, D.A.; Fu, F. Microorganisms and ocean global change. Nat. Microbiol. 2017, 2, 17058. [Google Scholar] [CrossRef]
- Jin, P.; Agustí, S. Fast adaptation of tropical diatoms to increased warming with trade-offs. Sci. Rep. 2018, 8, 17771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.N.; Goldman, J.A.L.; Kranz, S.A.; Tortell, P.D.; Morel, F.M.M. Slow carboxylation of RubisCO constrains the maximum rate of carbon fixation during Antarctic phytoplankton blooms. New Phytol. 2015, 205, 172–181. [Google Scholar] [CrossRef]
- Kranz, S.A.; Young, J.N.; Hopkinson, B.M.; Goldman, J.A.L.; Tortell, P.D.; Morel, F.M.M. Low temperature reduces the energetic requirement for the CO2 concentrating mechanism in diatoms. New Phytol. 2015, 205, 192–201. [Google Scholar] [CrossRef]
- Tilze, M.M.; Dubinsky, Z. Effects of temperature and day length on the mass balance of Antarctic phytoplankton. Polar Biol. 1998, 77, 35–42. [Google Scholar]
- Xu, G.; Liu, J.; Song, X.; Tan, M.; Ren, H.; Li, D.; Tan, Y.; Huang, L.; Li, G. Diel rhythm in photosynthetic performance of phytoplankton assemblages is predicted to be light-dependent from in situ and mesocosm chlorophyll fluorescence. J. Coast. Res. 2020, 104, 445–454. [Google Scholar] [CrossRef]
- Xu, G.; Liu, J.; Chen, B.; Li, G. Photoperiod mediates the differential physiological responses of smaller Thalassiosira pseudonana and larger Thalassiosira punctigera to temperature changes. J. Appl. Phycol. 2020, 32, 2863–2874. [Google Scholar] [CrossRef]
- Rost, B.; Wegener, A.L.; Riebesell, U.; Sültemeyer, D.F. Carbon acquisition of marine phytoplankton: Effect of photoperiod length. Limnol. Oceanogr. 2006, 51, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, R.L.; Dos Santos, M.E.V.; Pacheco, A.B.F.; Azevedo, S.M.F.D.E. Effects of light intensity and light quality on growth and circadian rhythm of saxitoxins production in Cylindrospermopsis raciborskii (Cyanobacteria). J. Plankton Res. 2009, 31, 481–488. [Google Scholar] [CrossRef]
- Prézelin, B.B. Diel periodicity in phytoplankton productivity. Hydrobiologia 1992, 238, 1–35. [Google Scholar] [CrossRef]
- Xie, Y.; Laws, E.A.; Yang, L.; Li, Q.-P.; Huang, B. Diel patterns of variable fluorescence and carbon fixation of picocyanobacteria Prochlorococcus-dominated phytoplankton in the South China Sea basin. Front. Microbiol. 2018, 9, 1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Talmy, D.; Campbell, D.A. Diatom growth responses to photoperiod and light are predictable from diel reductant generation. J. Phycol. 2017, 53, 95–107. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Song, X.; Jian, X.; Tang, C.; Campbell, D.A.; Li, G. High antioxidant capability interacts with respiration to mediate two Alexandrium species growth exploitation of photoperiods and light intensities. Harmful Algae 2019, 82, 26–34. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Gao, K. Effects of typhoon Kaemi on coastal phytoplankton assemblages in the South China Sea, with special reference to the effects of solar UV radiation. J. Geophys. Res. 2009, 114, G04029. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Woroch, A.D.; Donaher, N.A.; Cockshutt, A.M.; Campbell, D.A. A Hard day’s night: Diatoms continue recycling photosystem II in the dark. Front. Mar. Sci. 2016, 3, 218. [Google Scholar] [CrossRef] [Green Version]
- Talmy, D.; Blackford, J.; Hardman-Mountford, N.J.; Polimene, L.; Follows, M.J.; Geider, R.J. Flexible C:N ratio enhances metabolism of large phytoplankton when resource supply is intermittent. Biogeosciences 2014, 11, 4881–4895. [Google Scholar] [CrossRef] [Green Version]
- Mayfield, A.B.; Hsiao, Y.-Y.; Chen, H.-K.; Chen, C.-S. RubisCO expression in the dinoflagellate Symbiodinium sp. Is influenced by both photoperiod and endosymbiotic lifestyle. Mar. Biotechnol. 2014, 16, 371–384. [Google Scholar] [CrossRef]
- Paul, J.H.; Kang, J.B.; Tabita, F.R. Diel patterns of regulation of rbcL transcription in a cyanobacterium and a prymnesiophyte. Mar. Biotechnol. 2000, 2, 429–436. [Google Scholar] [CrossRef]
- Shi, X.; Li, L.; Guo, C.; Lin, X.; Li, M.; Lin, S. Rhodopsin gene expression regulated by the light dark cycle, light spectrum and light intensity in the dinoflagellate Prorocentrum. Front. Microbiol. 2015, 6, 555. [Google Scholar] [CrossRef]
- Garcés, E.; Delgado, M.; Masò, M.; Camp, J. Life history and in situ growth rates of Alexandrium taylori (Dinophyceae, Pyrrhophyta). J. Phycol. 1998, 34, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.M.; Swift, E.; Donaghay, P.L.; Rines, J.E.B. Small-Scale turbulence affects the division rate and morphology of two red-tide dinoflagellates. Harmful Algae 2003, 2, 183–199. [Google Scholar] [CrossRef]
- Anderson, D.M. Bloom dynamics of toxic Alexandrium species in the northeastern U.S. Limnol. Oceanogr. 1997, 42, 1009–1022. [Google Scholar] [CrossRef]
- Tsakalakis, I.; Pahlow, M.; Oschlies, A.; Blasius, B.; Ryabov, A.B. Diel light cycle as a key factor for modelling phytoplankton biogeography and diversity. Ecol. Model 2018, 384, 241–248. [Google Scholar] [CrossRef]
- Shatwell, T.; Köhler, J.; Nicklisch, A. Temperature and photoperiod interactions with phosphorus-limited growth and competition of two diatoms. PLoS ONE 2014, 9, e102367. [Google Scholar] [CrossRef] [Green Version]
- Shatwell, T.; Köhler, J.; Nicklisch, A. Temperature and photoperiod interactions with silicon-limited growth and competition of two diatoms. J. Plankton Res. 2013, 35, 957–971. [Google Scholar] [CrossRef] [Green Version]
- Shatwell, T.; Nicklisch, A.; Köhler, J. Temperature and photoperiod effects on phytoplankton growing under simulated mixed layer light fluctuations. Limnol. Oceanogr. 2012, 57, 541–553. [Google Scholar] [CrossRef]
- Nicklisch, A.; Shatwell, T.; Köhler, J. Analysis and modelling of the interactive effects of temperature and light on phytoplankton growth and relevance for the spring bloom. J. Plankton Res. 2008, 30, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2002, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Yoo, Y.D.; Kim, J.S.; Seong, K.A.; Kang, N.S.; Kim, T.H. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci. J. 2010, 45, 65–91. [Google Scholar] [CrossRef] [Green Version]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.T. Comparative physiological study of marine diatoms and dinoflagellates in relation to irradiance and cell size. I. Growth under continuous light. J. Phycol. 1978, 14, 396–402. [Google Scholar] [CrossRef]
- Chan, A.T. Comparative physiological study of marine diatoms and dinoflagellates in relation to irradiance and cell size. II. Relationship between photosynthesis, growth, and carbon/chlorophyll a ratio. J. Phycol. 1980, 16, 428–432. [Google Scholar] [CrossRef]
- Marañón, E.; Cermeño, P.; López-Sandoval, D.C.; Rodríguez-Ramos, T.; Sobrino, C.; Huete-Ortega, M.; Blanco, J.M.; Rodríguez, J. Unimodal size scaling of phytoplankton growth and the size dependence of nutrient uptake and use. Ecol. Lett. 2013, 16, 371–379. [Google Scholar] [CrossRef]
- Marañón, E.; Cermeno, P.; Rodriquez, J.; Zubkov, M.V.; Harris, R.P. Scaling of phytoplankton photosynthesis and cell size in the ocean. Limnol. Oceanogr. 2007, 52, 2190–2198. [Google Scholar] [CrossRef]
- Raven, J.A.; Kübler, J.E. New light on the scaling of metabolic rate with the size of algae. J. Phycol. 2002, 38, 11–16. [Google Scholar] [CrossRef]
- Key, T.; McCarthy, A.; Campbell, D.A.; Six, C.; Roy, S.; Finkel, Z.V. Cell size trade-offs govern light exploitation strategies in marine phytoplankton. Environ. Microbiol. 2010, 12, 95–104. [Google Scholar] [CrossRef]
- Ross, O.N.; Geider, R.J. New cell-based model of photosynthesis and photo-acclimation: Accumulation and mobilization of energy reserves in phytoplankton. Mar. Ecol. Prog. Ser. 2009, 383, 53–71. [Google Scholar] [CrossRef] [Green Version]
- Suggett, D.; MacIntyre, H.; Kana, T.; Geider, R. Comparing electron transport with gas exchange: Parameterising exchange rates between alternative photosynthetic currencies for eukaryotic phytoplankton. Aquat. Microbiol. Ecol. 2009, 56, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Halsey, K.H.; O’Malley, R.T.; Graff, J.R.; Milligan, A.J.; Behrenfeld, M.J. A common partitioning strategy for photosynthetic products in evolutionarily distinct phytoplankton species. New Phytol. 2013, 198, 1030–1038. [Google Scholar] [CrossRef]
- Lilly, E.L.; Halanych, K.M.; Anderson, D.M. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J. Phycol. 2007, 43, 1329–1338. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alperm, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [Green Version]
- Balech, E. The Genus Alexandrium Halim (Dinoflagellata); Sherkin Island Marine Station, Sherkin Island, Co.: Cork, Ireland, 1995. [Google Scholar]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G. Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: Synergistic action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Barton, A.D.; Finkel, Z.V.; Ward, B.A.; Johns, D.G.; Follows, M.J. On the roles of cell size and trophic strategy in North Atlantic diatom and dinoflagellate communities. Limnol. Oceanogr. 2013, 58, 254–266. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, J.; Yu, R.; Yan, T.; Wang, Y.; Kong, K.; Zhou, M. Roles of mixotrophy in blooms of different dinoflagellates: Implications from the growth experiment. Harmful Algae 2013, 30, 10–26. [Google Scholar] [CrossRef]
- Granum, E.; Kirkvold, S.; Myklestad, S.M. Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: Diel variations and effects of N depletion. Mar. Ecol. Prog. Ser. 2002, 242, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Campbell, D.A. Interactive effects of nitrogen and light on growth rates and RUBISCO content of small and large centric diatoms. Photosynth. Res. 2017, 131, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malerba, M.E.; Palacios, M.M.; Delgado, Y.M.P.; Beardall, J.; Marshall, D.J. Cell size, photosynthesis and the package effect: An artificial selection approach. New Phytol. 2018, 219, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Brand, L.E.; Guillard, R.R.L. The effects of continuous light and light intensity on the reproduction rates of twenty-two species of marine phytoplankton. J. Exp. Mar. Biol. Ecol. 1981, 50, 119–132. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Lemaire, S.D.; Crespo, J.L. Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 2012, 160, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Liu, S.; Huang, L.; Huang, H.; Lian, J.; Yan, Y.; Lin, S.-J. Diatom to dinoflagellate shift in the summer phytoplankton community in a bay impacted by nuclear power plant thermal effluent. Mar. Ecol. Prog. Ser. 2011, 424, 75–85. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve). Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, T.; Tan, Z.; Wang, L.; Zhou, M. Toxicity of four dinoflagellates Alexandrium species. Oceanol. Limnol. Sin. 2007, 38, 56–61. [Google Scholar]
- Sun, J.; Liu, D.; Qian, S. Study on phytoplankton biomass: Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanol. Sin. 1999, 21, 75–85. [Google Scholar]
- Genty, B.E.; Briantais, J.M.; Baker, N.R. Relative quantum efficiencies of the two photosystems of leaves in photorespiratory and non-photorespiratory conditions. Plant Physiol. Biochem. 1989, 28, 1–10. [Google Scholar]
- Van Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Jeffrey, S.; Humphrey, G. New spectrophotometric equations for determining chlorophylls a1, b1, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Savitch, L.V.; Butz, N.B. Photometric method for routine determination of kcat and carbamylation of RubisCO. Photosynth. Res. 1991, 28, 41–48. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ma, Q.; Xu, S.; Liu, W.; Ma, Z.; Ni, G. Opposite Growth Responses of Alexandrium minutum and Alexandrium catenella to Photoperiods and Temperatures. Plants 2021, 10, 1056. https://doi.org/10.3390/plants10061056
Li P, Ma Q, Xu S, Liu W, Ma Z, Ni G. Opposite Growth Responses of Alexandrium minutum and Alexandrium catenella to Photoperiods and Temperatures. Plants. 2021; 10(6):1056. https://doi.org/10.3390/plants10061056
Chicago/Turabian StyleLi, Ping, Qun Ma, Su Xu, Wenha Liu, Zengling Ma, and Guangyan Ni. 2021. "Opposite Growth Responses of Alexandrium minutum and Alexandrium catenella to Photoperiods and Temperatures" Plants 10, no. 6: 1056. https://doi.org/10.3390/plants10061056
APA StyleLi, P., Ma, Q., Xu, S., Liu, W., Ma, Z., & Ni, G. (2021). Opposite Growth Responses of Alexandrium minutum and Alexandrium catenella to Photoperiods and Temperatures. Plants, 10(6), 1056. https://doi.org/10.3390/plants10061056