Molecular Hydrogen Maintains the Storage Quality of Chinese Chive through Improving Antioxidant Capacity
Abstract
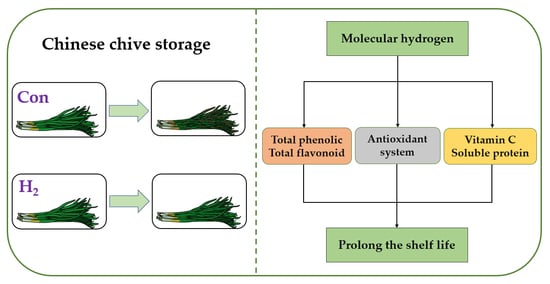
:1. Introduction
2. Results
2.1. Improvement of the Visual Quality of Chive during Storage in Response to Molecular Hydrogen
2.2. Changes of Total Phenolic and Flavonoid Contents
2.3. H2 Slowed Down the Decreased Vitamin C
2.4. Redox Balance Was Reestablished by H2
2.5. Antioxidant Enzymatic Activates Were Stimulated in the Presence of H2
2.6. Changes in Reduced Glutathione (GSH) and Glutathione Reductase (GR) Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Preparation of Hydrogen Gas
4.3. Determination of Decay Index and the Loss Ratio of Weight
4.4. Determination of Total Phenolic, Total Flavonoid
4.5. Laser Scanning Confocal Microscope
4.6. Determination of Hydrogen Peroxide (H2O2) Content
4.7. Assay of Thiobarbituric Acid Reactive Substances (TBARS) Content
4.8. Determination of 2,2-Diphenyl-1-Picrylhydrazyl Radical (DPPH) Scavenging Activity
4.9. Determination of Vitamin C Content
4.10. Assay of Antioxidant Enzyme Activity
4.11. Experimental Design
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APX | ascorbate peroxidase |
| ANOVA | one-way analysis of variance |
| BCA | bicinchoninic acid |
| CAT | catalase |
| Con | control |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FW | fresh weight |
| GSH | glutathione |
| GR | glutathione reductase |
| H2 | molecular hydrogen |
| H2DCFDA | 2′, 7′-Dichlorofluorescin diacetate |
| HPLC | high-performance liquid chromatography |
| H2O2 | hydrogen peroxide |
| LSCM | laser scanning confocal microscope |
| MCB | monochlorobimane |
| NBT | nitro blue tetrazolium |
| POD | guaiacol peroxidase |
| RH | relative humidity |
| ROS | reactive oxygen species |
| R.U. | relative units |
| SE | standard error |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
References
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Boichenko, E.A. Hydrogenase from isolated chloroplasts. Biokhimiya 1947, 12, 153–162. [Google Scholar]
- Renwick, G.M.; Giumarro, C.; Siegel, S.M. Hydrogen metabolism in higher plants. Plant Physiol. 1964, 39, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Q.; Zhang, M.Y.; Sun, X.J. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.B.; Sun, X.J. Hydrogen biology: It is just beginning. Chin. J. Biochem. Mol. Biol. 2019, 35, 1037–1050. [Google Scholar]
- Wang, Y.Q.; Liu, Y.H.; Wang, S.; Du, H.M.; Shen, W.B. Hydrogen agronomy: Research progress and prospects. J. Zhejiang Univ. Sci. B 2020, 21, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Zulficiar, F.; Hancock, J.T. Hydrogenases and the role of molecular hydrogen in plants. Plants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Li, L.N.; Lou, W.; Kong, L.S.; Shen, W.B. Hydrogen commonly applicable from medicine to agriculture: From molecular mechanisms to the field. Curr. Pharm. Des. 2021, 27, 747–759. [Google Scholar] [CrossRef]
- Xie, Y.J.; Mao, Y.; Lai, D.W.; Zhang, W.; Shen, W.B. H2 Enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.J.; Mao, Y.; Zhang, W.; Lai, D.W.; Wang, Q.Y.; Shen, W.B. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.J.; Cui, W.T.; Dai, C.; Zhu, K.K.; Zhang, J.; Wang, R.; La, H.G.; Li, X.; Shen, W.B. Involvement of hydrogen peroxide and heme oxygenase-1 in hydrogen gas-induced osmotic stress tolerance in alfalfa. Plant Growth Regul. 2016, 80, 215–223. [Google Scholar] [CrossRef]
- Felix, K.; Su, J.C.; Lu, R.F.; Zhao, G.; Cui, W.T.; Wang, R.; Mu, H.L.; Cui, J.; Shen, W.B. Hydrogen-induced tolerance against osmotic stress in alfalfa seedlings involves ABA signaling. Plant Soil 2019, 445, 409–423. [Google Scholar] [CrossRef]
- Cui, W.T.; Gao, C.Y.; Fang, P.; Lin, G.Q.; Shen, W.B. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013, 260, 715–724. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhao, G.; Cheng, P.F.; Yan, X.Y.; Li, Y.; Cheng, D.; Wang, R.; Chen, J.; Shen, W.B. Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas. Int. J. Food Prop. 2019, 22, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.L.; Li, P.X.; Wang, Y.N.; Gu, R.X. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014, 156, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.L.; Zhao, S.P.; Li, P.X.; Shen, W.B. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2018, 135, 123–130. [Google Scholar] [CrossRef]
- Wang, C.L.; Fang, H.; Gong, T.Y.; Zhang, J.; Niu, L.J.; Huang, D.J.; Huo, J.Q.; Liao, W.B. Hydrogen gas alleviates postharvest senescence of cut rose ’Movie star’ by antagonizing ethylene. Plant Mol. Biol. 2020, 102, 271–285. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.N.; Wang, S.; L, Y.H.; Zou, J.X.; Ding, W.J.; Du, H.M.; Shen, W.B. Magnesium hydride acts as a convenient hydrogen supply to prolong the vase life of cut roses by modulating nitric oxide synthesis. Postharvest Biol. Technol. 2021, 177, 111526. [Google Scholar] [CrossRef]
- Su, J.C.; Nie, Y.; Zhao, G.; Cheng, D.; Wang, R.; Chen, J.; Zhang, S.H.; Shen, W.B. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers. Postharvest Biol. Technol. 2019, 147, 148–155. [Google Scholar] [CrossRef]
- Li, L.N.; Liu, Y.H.; Wang, S.; Zou, J.X.; Ding, W.J.; Shen, W.B. Magnesium hydride-mediated sustainable hydrogen supply prolongs the vase life of cut carnation flowers via hydrogen sulfide. Front. Plant Sci. 2020, 11, 595376. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.L.; Zhang, L.J.; Chen, X.W.; Huang, Y.Q.; Sun, D.L.; Fang, F.; Guo, Z.P.; Yu, X.B. Carbon hollow nanobubbles on porous carbon nanofibers: An ideal host for high-performance sodium-sulfur batteries and hydrogen storage. Energy Storage Mater. 2018, 14, 314–323. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Cheng, P.F.; Wang, Y.Q.; Li, Y.; Su, J.C.; Chen, Z.P.; Yu, X.L.; Shen, W.B. Genetic elucidation of hydrogen signaling in plant osmotic tolerance and stomatal closure via hydrogen sulfide. Free. Radic. Biol. Med. 2020, 161, 1–14. [Google Scholar] [CrossRef]
- Imahori, Y.; Suzuki, Y.; Uemura, K.; Kishioka, I.; Fujiwara, H.; Ueda, Y.; Chachin, K. Physiological and quality responses of Chinese chive leaves to low oxygen atmosphere. Postharvest Biol. Technol. 2004, 31, 295–303. [Google Scholar] [CrossRef]
- Hu, G.H.; Lu, Y.H.; Wei, D.Z. Chemical characterization of Chinese chive seed (Allium tuberosum Rottl.). Food Chem. 2005, 99, 693–697. [Google Scholar] [CrossRef]
- Ishii, K.; Okubo, M. The keeping quality of Chinese chive (Allium tuberosum Rottler) by low temperature and seal-packaging with polyethylene bag. J. Jpn. Soc. Hortic. Sci. 1984, 53, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Borrel, A.; Carbonell, L.; Farràs, R.; PuigParcllada, P.; Tiburcio, A.F. Polyamines inhibit lipid peroxidation in senescing oat leaves. Physiol. Plant. 1997, 99, 385–390. [Google Scholar] [CrossRef]
- Wen-biao, S.; Mao-bing, Y.; Lang-lai, X.; Rong-xian, Z. Changes of ability of scavenging active oxygen during natural senescence of wheat flag leaves. J. Integr. Plant Biol. 1997, 39, 634–640. [Google Scholar]
- Rogers, H.; Munné-Bosch, S. Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: Similar but different. Plant Physiol. 2016, 171, 1560–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.E.; Liu, S.; Duan, X.M.; Zhang, C.; Wu, Z.H.; Liu, M.C.; Guo, S.G.; Zuo, J.H.; Wang, L.B. 6-Benzylaminopurine treatment maintains the quality of Chinese chive (Allium tuberosum Rottler ex Spreng.) by enhancing antioxidant enzyme activity. J. Integr. Agric. 2017, 16, 1968–1977. [Google Scholar] [CrossRef]
- Imahori, Y.; Suzuki, Y.; Kawagishi, M.; Ishimaru, M.; Ueda, Y.; Chachin, K. Physiological responses and quality attributes of Chinese chive leaves exposed to CO2-enriched atmospheres. Postharvest Biol. Technol. 2007, 46, 160–166. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A. Postharvest ozone application for the preservation of fruits and vegetables. Food Rev. Int. 2017, 33, 270–315. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Zhu, X.R.; Li, Y.B. Postharvest control of litchi fruit rot by Bacillus subtilis. LWT-Food Sci. Technol. 2001, 34, 430–436. [Google Scholar] [CrossRef]
- Wu, Z.F.; Tu, M.M.; Yang, X.P.; Xu, J.H.; Yu, Z.F. Effect of cutting on the reactive oxygen species accumulation and energy change in postharvest melon fruit during storage. Sci. Hortic. 2019, 257, 108752. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhao, F.; Yang, J.X.; Yang, H.Q. Nitric oxide alleviates lipid peroxidation induced by osmotic stress during senescence of detached leaves of Malus hupehensis Rehd. J. Hortic. Sci. Biotechnol. 2010, 85, 367–373. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.H.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.W.; Liang, D.; Ma, F.W. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.J.; Chen, X.; Chen, Z.S.Z.; Zhang, Z.K.; Li, T.T.; Qu, H.X.; Jiang, Y.M. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.W.; Dutta, P.; Dhua, R.S.; Dey, A. Changes in biochemical composition of mango in response to pre-harvest gibberellic acid spray. Agric. Conspec. Sci. 2014, 78, 331–335. [Google Scholar]
- Cantwell, M.I.; Thangaiah, A. Acceptable cooling delays for selected warm season vegetables and melons. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Postharvest Technology in the Global Market; Cantwell, M.I., Almeida, D.P.F., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2012; Volume 2, pp. 77–84. [Google Scholar]
- Zhang, X.Y.; Wei, J.Y.; Tian, J.Y.; Li, N.; Jia, L.; Shen, W.B.; Cui, J. Enhanced anthocyanin accumulation of immature radish microgreens by hydrogen-rich water under short wavelength light. Sci. Hortic. 2019, 247, 75–85. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martinez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Chen, Z.P.; Xie, Y.J.; Gu, Q.; Zhao, G.; Zhang, Y.H.; Cui, W.T.; Xu, S.; Wang, R.; Shen, W.B. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.P.; Cui, W.T.; Zhang, Y.H.; Hu, H.L.; Yu, X.L.; Wang, Q.Y.; Shen, W.B. Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol. Environ. Saf. 2018, 147, 861–871. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.P.; Yu, X.L.; Cui, W.T.; Pan, J.C.; Zhao, G.; Xu, S.; Wang, R.; Shen, W.B. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Su, J.C.; Zhang, Y.H.; Nie, Y.; Cheng, D.; Wang, R.; Hu, H.L.; Chen, J.; Zhang, J.F.; Du, Y.W.; Shen, W.B. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ. Exp. Bot. 2018, 147, 249–260. [Google Scholar] [CrossRef]
- Schaedle, M. Chloroplast glutathione reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef] [Green Version]
- In, B.C.; Ha, S.T.T.; Lee, Y.S.; Lim, J.H. Relationships between the longevity, water relations, ethylene sensitivity, and gene expression of cut roses. Postharvest Biol. Technol. 2017, 131, 74–83. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, K.; Kuang, Y.; Feng, L.; Liu, Y.; Wang, S.; Du, H.; Shen, W. Molecular Hydrogen Maintains the Storage Quality of Chinese Chive through Improving Antioxidant Capacity. Plants 2021, 10, 1095. https://doi.org/10.3390/plants10061095
Jiang K, Kuang Y, Feng L, Liu Y, Wang S, Du H, Shen W. Molecular Hydrogen Maintains the Storage Quality of Chinese Chive through Improving Antioxidant Capacity. Plants. 2021; 10(6):1095. https://doi.org/10.3390/plants10061095
Chicago/Turabian StyleJiang, Ke, Yong Kuang, Liying Feng, Yuhao Liu, Shu Wang, Hongmei Du, and Wenbiao Shen. 2021. "Molecular Hydrogen Maintains the Storage Quality of Chinese Chive through Improving Antioxidant Capacity" Plants 10, no. 6: 1095. https://doi.org/10.3390/plants10061095
APA StyleJiang, K., Kuang, Y., Feng, L., Liu, Y., Wang, S., Du, H., & Shen, W. (2021). Molecular Hydrogen Maintains the Storage Quality of Chinese Chive through Improving Antioxidant Capacity. Plants, 10(6), 1095. https://doi.org/10.3390/plants10061095