Chemical Composition, Allelopathic, Antioxidant, and Anti-Inflammatory Activities of Sesquiterpenes Rich Essential Oil of Cleome amblyocarpa Barratte & Murb.
Abstract
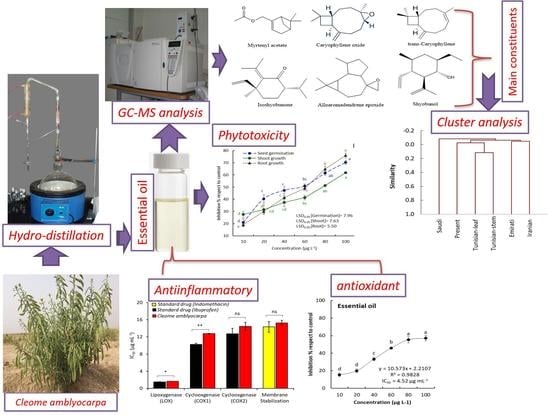
:1. Introduction
2. Results and Discussion
2.1. EO Composition of C. amblyocarpa
2.2. Chemometric Analysis of the EOs from Different C. amblyocarpa Ecospecies
2.3. Allelopathic Effect of the C. amblyocarpa EO
2.4. Antioxidant Activity of C. amblyocarpa EO
2.5. Anti-Inflammatory effect of C. amblyocarpa EO
3. Materials and Methods
3.1. Plant Materials
3.2. EO Extraction Analysis and Characterization
3.3. Allelopathic Bioassay
3.4. Antioxidant Activity
3.5. Anti-Inflammatory Activity Estimation
3.5.1. Membrane Stabilization Inhibition Assay
3.5.2. Lipoxygenase (LOX) Inhibition Assay
3.5.3. Cyclooxygenase (COX 1 and COX 2) Inhibition Assay
3.6. Treatment of Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Poliakoff, M.; Licence, P. Green chemistry. Nature 2007, 450, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Poliakoff, M.; Fitzpatrick, J.M.; Farren, T.R.; Anastas, P.T. Green chemistry: Science and politics of change. Science 2002, 297, 807–810. [Google Scholar] [CrossRef] [Green Version]
- Horváth, I.T.; Anastas, P.T. Innovations and green chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef] [Green Version]
- Elgamal, A.M.; Ahmed, R.F.; Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anticancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef] [PubMed]
- Assaeed, A.; Elshamy, A.; El Gendy, A.E.-N.; Dar, B.; Al-Rowaily, S.; Abd-ElGawad, A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Elshamy, A.; Essa, A.F.; El-Hawary, S.S.; El Gawad, A.M.A.; Kubacy, T.M.; El-Khrisy, E.E.-D.A.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crop. Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Aouni, M.; Anthonissen, R.; Verschaeve, L. Investigation on the genotoxicity of extracts from Cleome amblyocarpa Barr. and Murb, an important Tunisian medicinal plant. South Afr. J. Bot. 2013, 84, 102–103. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Gawad, A.M.; El Gendy, A.E.-N.; El-Amier, Y.; Gaara, A.; Omer, E.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018, 15, e1700438. [Google Scholar] [CrossRef]
- IPNI. The International Plant Names Index; The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens: Richmond, UK, 2012. [Google Scholar]
- IUCN. A Guide to Medicinal Plants in North Africa; IUCN: Barcelona, Spain, 2005. [Google Scholar]
- Bouriche, H.; Arnhold, J. Effect of Cleome arabica leaf extract treated by naringinase on human neutrophil chemotaxis. Nat. Prod. Commun. 2010, 5, 415–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlifi, A.; Pecio, Ł.; Lobo, J.C.; Melo, D.; Ayache, S.B.; Flamini, G.; Oliveira, M.B.P.; Oleszek, W.; Achour, L. Leaves of Cleome amblyocarpa Barr. and Murb. and Cleome arabica L.: Assessment of nutritional composition and chemical profile (LC-ESI-MS/MS), anti-inflammatory and analgesic effects of their extracts. J. Ethnopharmacol. 2021, 269, 113739. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Al-Karmalawy, A.A.; El-Amier, Y.A.; Ashour, A. Molecular docking reveals the potential of Cleome amblyocarpa isolated compounds to inhibit COVID-19 virus main protease. New J. Chem. 2020, 44, 16752–16758. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Kattab, A.M.; Bodige, S.G.; Mao, Y.; Minter, D.E.; Reinecke, M.G.; Watson, W.H.; Mabry, T.J. 15α-Acetoxycleomblynol A from Cleome amblyocarpa. J. Nat. Prod. 2001, 64, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Seglab, F.; Hamia, C.; Khacheba, I.; Djeridane, A.; Yousfi, M. High in vitro antioxidant capacities of Algerian Cleome arabica leaves’ extracts. Phytothérapie 2021, 19, 16–24. [Google Scholar] [CrossRef]
- Takhi, D.; Ouinten, M.; Yousfi, M. Study of antimicrobial activity of secondary metabolites extracted from spontaneous plants from the area of Laghouat, Algeria. Adv. Environ. Biol. 2011, 5, 469–477. [Google Scholar]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018, 15, e1800392. [Google Scholar] [CrossRef] [PubMed]
- Muhaidat, R.; Al-Qudah, M.A.; Samir, O.; Jacob, J.H.; Hussein, E.; Al-Tarawneh, I.N.; Bsoul, E.; Orabi, S.T.A. Phytochemical investigation and in vitro antibacterial activity of essential oils from Cleome droserifolia (Forssk.) Delile and C. trinervia Fresen. (Cleomaceae). South Afr. J. Bot. 2015, 99, 21–28. [Google Scholar] [CrossRef]
- Ndungu, M.; Lwande, W.; Hassanali, A.; Moreka, L.; Chhabra, S.C. Cleome monophylla essential oil and its constituents as tick (Rhipicephalus appendiculatus) and maize weevil (Sitophilus zeamais) repellents. Entomol. Exp. Appl. 1995, 76, 217–222. [Google Scholar] [CrossRef] [Green Version]
- McNeil, M.J.; Porter, R.B.; Williams, L.A. Chemical composition and biological activity of the essential oil from Jamaican Cleome serrata. Nat. Prod. Commun. 2012, 7, 1231–1232. [Google Scholar] [CrossRef] [Green Version]
- Sodeifian, G.; Saadati Ardestani, N.; Sajadian, S.A.; Ghorbandoost, S. Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: Experimental, response surface and grey wolf optimization methodology. J. Supercrit. Fluids 2016, 114, 55–63. [Google Scholar] [CrossRef]
- Shahin, S.; Kurup, S.; Cheruth, A.-J.; Salem, M. Chemical composition of Cleome amblyocarpa Barr. & Murb. essential oils under different irrigation levels in sandy soils with antioxidant activity. J. Essent. Oil Bear. Plants 2018, 21, 1235–1256. [Google Scholar]
- Rassouli, E.; Dadras, O.G.; Bina, E.; Asgarpanah, J. The essential oil composition of Cleome brachycarpa Vahl ex DC. J. Essent. Oil Bear. Plants 2014, 17, 158–163. [Google Scholar] [CrossRef]
- Al-Humaidia, J.Y.; Al-Qudah, M.A.; Al-Saleem, M.S.; Alotaibi, S.M. Antioxidant activity and chemical composition of essential oils of selected Cleome species growing in Saudi Arabia. Jordan J. Chem. 2019, 14, 29–37. [Google Scholar]
- Abd El-Gawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.; Abd El-Gawad, A.M.; El-Amier, Y.A.; El Gendy, A.; Al-Rowaily, S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Hassan, E.M.; El Gendy, A.E.-N.G.; Abd El-Gawad, A.M.; Elshamy, A.I.; Farag, M.A.; Alamery, S.F.; Omer, E.A. Comparative chemical profiles of the essential oils from different varieties of Psidium guajava L. Molecules 2021, 26, 119. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Abd-El-Gawad, A.M.; Elshamy, A.; Al-Rowaily, S.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium Curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Badry, M.A.; Eldahshan, O.A.; Singab, A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2020, 34, 1–4. [Google Scholar] [CrossRef]
- Sam, L.N.; Huong, L.T.; Minh, P.N.; Vinh, B.T.; Dai, D.N.; Setzer, W.N.; Ogunwande, I.A. Chemical composition and antimicrobial activity of the rhizome essential oil of Curcuma sahuynhensis from Vietnam. J. Essent. Oil Bear. Plants 2020, 23, 803–809. [Google Scholar] [CrossRef]
- Khlifi, A.; Chrifa, A.B.; Lamine, J.B.; Thouri, A.; Adouni, K.; Flamini, G.; Oleszek, W.; Achour, L. Gas chromatography-mass spectrometry (GM-MS) analysis and biological activities of the aerial part of Cleome amblyocarpa Barr. and Murb. Environ. Sci. Pollut. Res. 2020, 27, 22670–22679. [Google Scholar] [CrossRef]
- McNeil, M.J.; Porter, R.B.; Williams, L.A.; Rainford, L. Chemical composition and antimicrobial activity of the essential oils from Cleome spinosa. Nat. Prod. Commun. 2010, 5, 1301–1306. [Google Scholar] [CrossRef] [Green Version]
- Balogun, O.S.; Ajayi, O.S.; Adeleke, A.J. Hexahydrofarnesyl acetone-rich extractives from Hildegardia barteri. J. Herbs Spices Med. Plants 2017, 23, 393–400. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; El Gendy, A.; Elshamy, A.; Omer, E. Chemical composition of the essential oil of Trianthema portulacastrum L. Aerial parts and potential antimicrobial and phytotoxic activities of its extract. J. Essent. Oil Bear. Plants 2016, 19, 1684–1692. [Google Scholar] [CrossRef]
- Fayed, E.M.; Abd EI-Gawad, A.M.; Elshamy, A.I.; El-Halawany, E.S.F.; EI-Amier, Y.A. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021, 18, e2000914. [Google Scholar] [CrossRef]
- Saleh, I.; Abd El-Gawad, A.; El Gendy, A.E.-N.; Abd El Aty, A.; Mohamed, T.; Kassem, H.; Aldosri, F.; Elshamy, A.; Hegazy, M.-E.F. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants 2020, 9, 716. [Google Scholar] [CrossRef]
- Ladhari, A.; Gaaliche, B.; Zarrelli, A.; Ghannem, M.; Ben Mimoun, M. Allelopathic potential and phenolic allelochemicals discrepancies in Ficus carica L. cultivars. South Afr. J. Bot. 2020, 130, 30–44. [Google Scholar] [CrossRef]
- Chon, S.U.; Kim, Y.M.; Lee, J.C. Herbicidal potential and quantification of causative allelochemicals from several Compositae weeds. Weed Res. 2003, 43, 444–450. [Google Scholar] [CrossRef]
- Ladhari, A.; Omezzine, F.; DellaGreca, M.; Zarrelli, A.; Zuppolini, S.; Haouala, R. Phytotoxic activity of Cleome arabica L. and its principal discovered active compounds. South Afr. J. Bot. 2013, 88, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Ladhari, A.; Tufano, I.; DellaGreca, M. Influence of new effective allelochemicals on the distribution of Cleome arabica L. community in nature. Nat. Prod. Res. 2020, 34, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Narouei, M.; Majrohi, A.A.; Chamanabad, H.R.M. Chemical constituents and phytotoxic activity of the essential oil of Acroptilon repens (L.) Dc from Iran. J. Essent. Oil Bear. Plants 2012, 15, 943–948. [Google Scholar] [CrossRef]
- de Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [Green Version]
- Mancini, E.; Arnold, N.A.; De Martino, L.; De Feo, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon. Molecules 2009, 14, 4725–4736. [Google Scholar] [PubMed] [Green Version]
- Abd El-Gawad, A.M.; Elshamy, A.; El Gendy, A.E.-N.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef]
- Mancini, E.; De Martino, L.; Malova, H.; De Feo, V. Chemical composition and biological activities of the essential oil from Calamintha nepeta plants from the wild in southern Italy. Nat. Prod. Commun. 2013, 8, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.L.; Wen, J.; Guo, Q.F. Mechanism and active variety of allelochemicals. Acta Bot. Sin. 2004, 46, 757–766. [Google Scholar]
- El-Kashak, W.A.; Elshamy, A.I.; Mohamed, T.A.; El Gendy, A.E.-N.G.; Saleh, I.A.; Umeyama, A. Rumpictuside A: Unusual 9,10-anthraquinone glucoside from Rumex pictus Forssk. Carbohydr. Res. 2017, 448, 74–78. [Google Scholar] [CrossRef]
- de Santana Souza, M.T.; Almeida, J.R.G.d.S.; de Souza Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.F.; dos Santos, M.R.V.; Quintans-Júnior, L.J. Structure–activity relationship of terpenes with anti-inflammatory profile—A systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Ribeiro-Filho, J.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Júnior, L.J.Q.; de Sousa Araújo, A.A. Anti-inflammatory activity of the essential oil obtained from Ocimum basilicum complexed with β-cyclodextrin (β-CD) in mice. Food Chem. Toxicol. 2017, 109, 836–846. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Prakash, O.; Srivastava, R.; Pant, A. Chemical composition, in vitro antioxidant, anti-inflammatory and antifeedant properties in the essential oil of Asian marsh weed Limnophila indica L. (Druce). J. Pharmacogn. Phytochem. 2019, 8, 1689–1694. [Google Scholar]
- De Cássia Da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. Sesquiterpenes from essential oils and anti-inflammatory activity. Nat. Prod. Commun. 2015, 10, 1767–1774. [Google Scholar] [CrossRef] [Green Version]
- Chavan, M.; Wakte, P.; Shinde, D. Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Passos, G.F.; Fernandes, E.S.; da Cunha, F.M.; Ferreira, J.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J. Ethnopharmacol. 2007, 110, 323–333. [Google Scholar] [CrossRef]
- Medeiros, R.; Passos, G.; Vitor, C.; Koepp, J.; Mazzuco, T.; Pianowski, L.; Campos, M.; Calixto, J. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Foddai, M.; Marchetti, M.; Ruggero, A.; Juliano, C.; Usai, M. Evaluation of chemical composition and anti-inflammatory, antioxidant, antibacterial activity of essential oil of Sardinian Santolina corsica Jord. & Fourr. Saudi J. Biol. Sci. 2019, 26, 930–937. [Google Scholar]
- Touaibia, M. Composition and anti-inflammatory effect of the common myrtle (Myrtus communis L.) essential oil growing wild in Algeria. Phytotherapie 2020, 18, 156–161. [Google Scholar] [CrossRef]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974. [Google Scholar]
- Boulos, L. Flora of Egypt; All Hadara Publishing: Cairo, Egypt, 1995; Volume 1. [Google Scholar]
- Abd El-Gawad, A.; Elshamy, A.; El Gendy, A.E.-N.; Gaara, A.; Assaeed, A. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. Flavour Fragr. J. 2010, 25, 219–312. [Google Scholar] [CrossRef]
- Shinde, U.; Phadke, A.; Nair, A.; Mungantiwar, A.; Dikshit, V.; Saraf, M. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N.; Murray, M. Using N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) to assay cyclooxygenase activity In Vitro. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 129–140. [Google Scholar] [CrossRef]







| No. | RT a | RC b % | Compound Name | MF c | KILit d | KIExp e | Identification f |
|---|---|---|---|---|---|---|---|
| Oxygenated monoterpenes | |||||||
| 1 | 6.60 | 0.50 | 6-Camphenol | C10H16O | 1113 | 1113 | KI, MS |
| 2 | 8.94 | 0.13 | Eucalyptol | C10H18O | 1031 | 1032 | KI, MS |
| 3 | 10.24 | 0.54 | Camphor | C10H16O | 1146 | 1165 | KI, MS |
| 4 | 11.00 | 0.45 | Isopulegol | C10H18O | 1149 | 1150 | KI, MS |
| 5 | 12.76 | 1.12 | Borneol | C10H18O | 1169 | 1169 | KI, MS |
| 6 | 11.99 | 0.25 | 2-ethyl-exo-Fenchol | C10H18O | 1297 | 1299 | KI, MS |
| 7 | 26.43 | 5.73 | Myrtenyl acetate | C12H18O2 | 1326 | 1324 | KI, MS |
| Sesquiterpenes hydrocarbons | |||||||
| 8 | 16.34 | 0.13 | Silphiperfol-5,7(14)-diene | C15H22 | 1360 | 1361 | KI, MS |
| 9 | 17.28 | 0.98 | α-Copaene | C15H24 | 1376 | 1378 | KI, MS |
| 10 | 17.48 | 1.01 | β-Maaliene | C15H24 | 1380 | 1381 | KI, MS |
| 11 | 17.70 | 0.47 | Berkheyaradulene | C15H24 | 1388 | 1389 | KI, MS |
| 12 | 18.68 | 3.45 | trans-Caryophyllene | C15H24 | 1408 | 1410 | KI, MS |
| 13 | 18.79 | 0.29 | Widdrene | C15H24 | 1431 | 1430 | KI, MS |
| 14 | 19.22 | 0.46 | β-Humulene | C15H24 | 1438 | 1436 | KI, MS |
| 15 | 19.85 | 0.49 | β-Farnesene | C15H24 | 1442 | 1443 | KI, MS |
| 16 | 20.47 | 0.53 | γ-Muurolene | C15H24 | 1479 | 1477 | KI, MS |
| 17 | 20.78 | 0.23 | ar-Curcumene | C15H22 | 1480 | 1481 | KI, MS |
| 18 | 20.86 | 0.54 | Valencene | C15H24 | 1496 | 1496 | KI, MS |
| 19 | 20.94 | 0.65 | α-Selinene | C15H24 | 1498 | 1497 | KI, MS |
| 20 | 21.20 | 2.30 | α-Muurolene | C15H24 | 1500 | 1500 | KI, MS |
| 21 | 21.78 | 1.17 | α-Bulnesene | C15H24 | 1509 | 1510 | KI, MS |
| 22 | 22.00 | 1.02 | γ-Cadinene | C15H24 | 1513 | 1514 | KI, MS |
| 23 | 23.55 | 0.42 | trans-Calamenene | C15H22 | 1522 | 1520 | KI, MS |
| 24 | 32.80 | 1.01 | α-Guaiene | C15H24 | 1600 | 1601 | KI, MS |
| Oxygenated sesquiterpenes | |||||||
| 25 | 20.21 | 0.13 | β-Cubebene | C15H24O | 1388 | 1389 | KI, MS |
| 26 | 21.53 | 1.53 | trans-α-Bisabolene epoxide | C15H24O | 1506 | 1508 | KI, MS |
| 27 | 21.67 | 1.54 | trans-Nerolidol | C15H24O | 1531 | 1533 | KI, MS |
| 28 | 23.17 | 4.52 | Isoshyobunone | C15H24O | 1571 | 1570 | KI, MS |
| 29 | 23.48 | 0.66 | Spathulenol | C15H24O | 1577 | 1579 | KI, MS |
| 30 | 23.78 | 36.01 | Caryophyllene oxide | C15H24O | 1582 | 1584 | KI, MS |
| 31 | 24.22 | 0.86 | Humulene oxide | C15H24O | 1608 | 1609 | KI, MS |
| 32 | 24.45 | 0.83 | Junenol | C15H26O | 1619 | 1621 | KI, MS |
| 33 | 24.53 | 0.56 | Citronellyl valerate | C15H28O2 | 1625 | 1626 | KI, MS |
| 34 | 24.63 | 1.18 | Nerolidol-epoxyacetate | C17H28O4 | 1638 | 1637 | KI, MS |
| 35 | 24.88 | 0.38 | tau-Cadinol | C15H26O | 1640 | 1639 | KI, MS |
| 36 | 25.34 | 6.17 | Alloaromadendrene epoxide | C15H24O | 1641 | 1643 | KI, MS |
| 37 | 25.57 | 0.71 | α-Eudesmol | C15H26O | 1653 | 1655 | KI, MS |
| 38 | 25.80 | 0.32 | Aromadendrene oxide-(2) | C15H24O | 1678 | 1680 | KI, MS |
| 39 | 25.99 | 4.19 | Shyobunol | C15H24O | 1688 | 1691 | KI, MS |
| 40 | 26.79 | 0.49 | β-Santalol | C15H24O | 1738 | 1735 | KI, MS |
| 41 | 29.32 | 0.54 | Xanthorrhizol | C15H22O | 1753 | 1752 | KI, MS |
| Diterpenes hydrocarbons | |||||||
| 42 | 33.47 | 0.22 | Geranyl-α-terpinene | C20H32 | 1874 | 1872 | KI, MS |
| Oxygenated diterpenes | |||||||
| 43 | 37.49 | 0.17 | Phytol | C20H40O | 1942 | 1944 | KI, MS |
| Carotenoid derived compounds | |||||||
| 44 | 14.56 | 1.39 | Theaspirane A | C13H22O | 1305 | 1307 | KI, MS |
| 45 | 14.87 | 1.64 | Dihydroedulan II | C13H22O | 1318 | 1316 | KI, MS |
| Apocarotenoid derived compounds | |||||||
| 46 | 30.87 | 7.92 | Hexahydrofarnesyl acetone | C18H36O | 1835 | 1837 | KI, MS |
| Other compounds | |||||||
| 47 | 13.87 | 1.16 | p-Isopropyl-benzaldehyde | C10H12O | 1239 | 1240 | KI, MS |
| 48 | 38.11 | 0.18 | 9,12-Octadecadienoic acid | C18H32O2 | 2085 | 2085 | KI, MS |
| Total | 97.17 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Elgamal, A.M.; EI-Amier, Y.A.; Mohamed, T.A.; El Gendy, A.E.-N.G.; I. Elshamy, A. Chemical Composition, Allelopathic, Antioxidant, and Anti-Inflammatory Activities of Sesquiterpenes Rich Essential Oil of Cleome amblyocarpa Barratte & Murb. Plants 2021, 10, 1294. https://doi.org/10.3390/plants10071294
Abd-ElGawad AM, Elgamal AM, EI-Amier YA, Mohamed TA, El Gendy AE-NG, I. Elshamy A. Chemical Composition, Allelopathic, Antioxidant, and Anti-Inflammatory Activities of Sesquiterpenes Rich Essential Oil of Cleome amblyocarpa Barratte & Murb. Plants. 2021; 10(7):1294. https://doi.org/10.3390/plants10071294
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Abdelbaset M. Elgamal, Yasser A. EI-Amier, Tarik A. Mohamed, Abd El-Nasser G. El Gendy, and Abdelsamed I. Elshamy. 2021. "Chemical Composition, Allelopathic, Antioxidant, and Anti-Inflammatory Activities of Sesquiterpenes Rich Essential Oil of Cleome amblyocarpa Barratte & Murb." Plants 10, no. 7: 1294. https://doi.org/10.3390/plants10071294
APA StyleAbd-ElGawad, A. M., Elgamal, A. M., EI-Amier, Y. A., Mohamed, T. A., El Gendy, A. E. -N. G., & I. Elshamy, A. (2021). Chemical Composition, Allelopathic, Antioxidant, and Anti-Inflammatory Activities of Sesquiterpenes Rich Essential Oil of Cleome amblyocarpa Barratte & Murb. Plants, 10(7), 1294. https://doi.org/10.3390/plants10071294