Chemical Composition and Cosmeceutical Potential of the Essential Oil of Oncosiphon suffruticosum (L.) Källersjö
Abstract
:1. Introduction
2. Results and Discussion
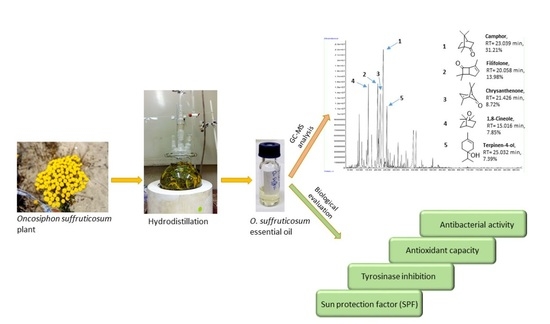
2.1. Chemical Composition of O. suffruticosum Essential Oil
2.2. Antibacterial Activity: Minimum Inhibitory Concentration (MIC) Using the Broth Microdilution Method
2.3. Antioxidant Capacities
2.4. Tyrosinase Inhibition
2.5. Sun Protection Factor (SPF)
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Essential Oil
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.4. Antibacterial Assay
3.4.1. Micro-Organisms
3.4.2. Preparation of the Media
3.4.3. Broth Microdilution Susceptibility Assay
3.5. Antioxidant Capacity Assays
3.5.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
3.5.2. 2,2′-Azino-bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Assay
3.5.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.5.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Antityrosinase Assay
3.6.1. Essential Oils Samples and Positive Control Preparation
3.6.2. Tyrosinase Inhibition Assay
3.7. Sun Protection Factor (SPF)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Properzi, A.; Angelini, P.; Bertuzzi, G.; Venanzoni, R. Some biological activities of essential oils. Med. Aromat. Plants 2013, 2, 136. [Google Scholar]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Tu, P.T.B.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [Green Version]
- Manosroi, A.; Manosroi, J. Free radical scavenging and tyrosinase inhibition activity of aromatic volatile oil from Thai medicinal plants for cosmetic uses. Acta Hortic. 2005, 680, 97–100. [Google Scholar] [CrossRef]
- Salleh, W.W.; Ahmad, F.; Yen, K.H.; Zulkifli, R.M. Chemical compositions and biological activities of essential oils of Beilschmiedia glabra. Nat. Prod. Commun. 2015, 10, 1297–1300. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, R.; Michael Jubeli, R.; El Beyrouthy, M.; Baillet Guffroy, A.; Rizk, T.; Tfayli, A.; Lteif, R. Phytochemical screening and antityrosinase activity of carvacrol, thymoquinone, and four essential oils of Lebanese plants. J. Cosmet. Dermatol. 2018, 18, 944–952. [Google Scholar] [CrossRef]
- IRC Conference on Science, Engineering and Technology. Available online: http://ircset.org/anand/2017papers/IRC-SET_2017_paper_S3-3.pdf (accessed on 4 June 2021).
- Mali, S.S.; Killedar, S.G. Formulation and in vitro evaluation of gel for SPF determination and free radical scavenging activity of turpentine and lavender oil. Pharma Innov. J. 2018, 7, 85–90. [Google Scholar]
- Androutsopoulou, C.; Christopoulou, S.D.; Hahalis, P.; Kotsalou, C.; Lamari, F.N.; Vantarakis, A. Evaluation of essential oils and extracts of rose geranium and rose petals as natural preservatives in terms of toxicity, antimicrobial, and antiviral activity. Pathogens 2021, 10, 494. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; van Staden, A.B.; Hosenally, M.; Mahomoodally, M. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics-a critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Western Cape Government-Biodiversity. Available online: https://www.westerncape.gov.za/text/2005/12/04_biodiversity_optimised.pdf. (accessed on 13 April 2018).
- Van Vuuren, S.F. Antimicrobial activity of South African medicinal plants. J. Ethnopharmacol. 2008, 119, 462–472. [Google Scholar] [CrossRef]
- Kolokoto, R.; Magee, A.R. Cape stinkweeds: Taxonomy of Oncosiphon (Anthemideae, Asteraceae). S. Afr. J. Bot. 2018, 117, 57–70. [Google Scholar] [CrossRef]
- Magee, A.R. Oncosiphon suffructicosum. 2011. Available online: http://pza.sanbi.org/oncosiphon-suffructicosum (accessed on 13 April 2018).
- Scott, G.; Springfield, E.P.; Coldrey, N. A pharmacognostical study of 26 South African plant species used as traditional medicines. Pharm. Biol. 2004, 42, 186–213. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014, 153, 61–84. [Google Scholar] [CrossRef] [Green Version]
- Goudjil, M.B.; Zighmi, S.; Hamada, D.; Mahcene, Z.; Bencheikh, S.E.; Ladjel, S. Biological activities of essential oils extracted from Thymus capitatus (Lamiaceae). S. Afr. J. Bot. 2020, 128, 274–282. [Google Scholar] [CrossRef]
- Van Wyk, B.E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Standards and Technologies. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 16 October 2019).
- Hulley, I.M.; Sadgrove, N.J.; Tilney, P.M.; Özek, G.; Yur, S.; Özek, T.; Başer, K.H.C.; van Wyk, B.E. Essential oil composition of Pentzia incana (Asteraceae), an important natural pasture plant in the Karoo region of South Africa. Afr. J. Range Forage Sci. 2018, 35, 137–145. [Google Scholar] [CrossRef]
- Hulley, I.M.; Özek, O.G.; Sadgrove, N.J.; Tilney, P.M.; Özek, T.; Başer, K.H.C.; van Wyk, B.E. Essential oil composition of a medicinally important Cape species: Pentzia punctata (Asteraceae). S. Afr. J. Bot. 2019, 127, 208–212. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Wózniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy: Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press: San Diego, CA, USA, 2017; p. 251. [Google Scholar]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [Green Version]
- Garg, C.; Khurana, P.; Garg, M. Molecular mechanisms of skin photoaging and plant inhibitors. Int. J. Green Pharm. 2017, 11, 17–33. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; Intech: Rijeka, Croatia, 2019; pp. 1–29. [Google Scholar]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 3, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Saeio, K.; Chaiyana, W.; Okonogi, S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov. Ther. 2011, 5, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mbanga, L.; Mulenga, M.; Mpiana, P.T.; Bokolo, K.; Mumbwa, M.; Mvingu, K. Determination of sun protection factor (SPF) of some body creams and lotions marketed in Kinshasa by ultraviolet spectrophotometry. Int. J. Adv. Res. Chem. Sci. 2014, 1, 7–13. [Google Scholar]
- Tobin, D. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohani, A.; Mishra, A.K.; Verma, A. Cosmeceutical potential of geranium and calendula essential oil: Determination of antioxidant activity and in vitro sun protection factor. J. Cosmet. Dermatol. 2019, 18, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinação do fato de proteção solar por espectrofotometrica. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sun screening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Gaikwad, M.; Bhandare, S. Determination and comparison of in vitro SPF of topical formulation containing Lutein ester from Tagetes erecta L. flowers, Moringa oleifera Lam seed oil and Moringa oleifera Lam seed oil containing Lutein ester. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1220–1224. [Google Scholar]
- Imam, S.; Azhar, I.; Mahmood, Z.A. In-vitro evaluation of sun protection factor of a cream formulation prepared from extracts of Musa accuminata (L.), Psidium gujava (L.) and Pyrus communis (L.). Asian J. Pharm. Clin. Res. 2015, 8, 234–237. [Google Scholar]
- Khor, P.Y.; Na’im Mohamed, F.S.; Ramli, I.; Nor, N.F.A.M.; Razali, S.K.C.M.; Zainuddin, J.A.; Jaafar, N.S.M. Phytochemical, antioxidant and photo-protective activity study of Bunga Kantan (Etlingera elatior) essential oil. J. Appl. Pharm. Sci. 2017, 7, 209–213. [Google Scholar]
- Agarwal, R.; Pant, A.K.; Prakash, O. Chemical composition and biological activities of essential oils of Cinnamomum tamala, Cinnamomum zeylenicum and Cinnamomum camphora growing in Uttarakhand. In Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives; Khemani, L.D., Srivastava, M.M., Srivastava, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–92. [Google Scholar]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, B.; Kedia, A.; Singh, A.; Yadav, S.; Singh, A.; Yadav, A.; Dubey, N.K. Antifungal, antiaflatoxin and antioxidant activity of plant essential oils and their in vivo efficacy in protection of chickpea seeds. J. Food Qual. 2016, 39, 36–44. [Google Scholar] [CrossRef]
- Amri, I.; De Martino, L.; Marandino, A.; Lamia, H.; Mohsen, H.; Scandolera, E.; De Feo, V.; Mancini, E. Chemical composition and biological activities of the essential oil from Artemisia herba-alba growing wild in Tunisia. Nat. Prod. Commun. 2013, 8, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzidi, N.; Mederbal, K.; Raho, B. Antioxidant activity of essential oil of Artemisia herba alba. J. Appl. Environ. Biol. Sci. 2016, 6, 59–65. [Google Scholar]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six Algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef] [Green Version]
- Derwich, E.; Benziane, Z.; Boukir, A. GC/MS analysis of volatile constituents and antibacterial activity of the essential oil of the leaves of Eucalyptus globulus in Atlas Median from Morocco. Adv. Nat. Appl. Sci. 2009, 3, 305–314. [Google Scholar]
- Barbosa, L.C.; Filomeno, C.A.; Teixeira, R.R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerbi, A.; Derbali, A.; Elfeki, A.; Kammoun, M. Essential oil composition and biological activities of Eucalyptus globulus leaves extracts from Tunisia. J. Essent. Oil Bear. Plants 2017, 20, 438–448. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef] [PubMed]
- Mickiene, R.; Bakutis, B.; Baliukoniene, V. Antimicrobial activity of two essential oils. Ann. Agric. Environ. Med. 2011, 18, 139–144. [Google Scholar] [PubMed]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef]
- Europarådet; European Pharmacopoeia Commission. European Pharmacopoeia; Maisonneuve: Sainte-Ruffine, France, 1975; Volume 3, pp. 68–80. [Google Scholar]
- Kuiate, J.R.; Amvam Zollo, P.H.; Nguefa, E.H.; Bessière, J.M.; Lamaty, G.; Menut, C. Composition of the essential oils from the leaves of Microglossa pyrifolia (Lam.) O. Kuntze and Helichrysum odoratissimum (L.) Less. growing in Cameroon. Flavour Fragr. J. 1999, 14, 82–84. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Lourens, A.C.U.; Reddy, D.; Başer, K.H.C.; Viljoen, A.M.; Van Vuuren, S.F. In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 2004, 95, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. Lebensm.-Wiss. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.A.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 238, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of oxidative stress and skin aging-related enzymes by prenylated chalcones and other flavonoids from Helichrysum teretifolium. Molecules 2015, 20, 7143–7155. [Google Scholar] [CrossRef]
- Cui, H.; Duan, F.; Jia, S.; Cheng, F.; Yuan, K. Antioxidant and tyrosinase inhibitory activities of seed oils from Torreya grandis Fort. ex Lindl. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar]


| RT (Min) | Component Code | Mass Spectral Matching | Composition (%) | Experimental RI | Literature RI | Identification |
|---|---|---|---|---|---|---|
| 9.214 | 1 | α-Pinene | 0.80 | 935 | 939 A | RI, MS |
| 9.981 | 2 | Camphene | 2.17 | 950 | 950 B | RI, MS |
| 11.374 | 3 | Sabinene | 0.54 | 974 | 973 B | RI, MS |
| 13.928 | 4 | α-Terpinene | 0.71 | 1016 | 1017 B | RI, MS |
| 14.508 | 5 | p-Cymene | 2.45 | 1026 | 1024 B | RI, MS |
| 15.016 | 6 | 1,8-Cineole | 7.85 | 1035 | 1032B | RI, MS |
| 16.710 | 7 | γ-Terpinene | 1.48 | 1061 | 1060 B | RI, MS |
| 20.058 | 8 | Filifolone | 13.98 | 1109 | 1109Wb | RI |
| 20.372 | 9 | Unknown | 2.56 | 1114 | - | - |
| 20.560 | 10 | Unknown | 2.03 | 1117 | - | - |
| 21.426 | 11 | Chrysanthenone | 8.72 | 1131 | 1125B | RI, MS |
| 23.039 | 12 | Camphor | 31.21 | 1155 | 1156Wb | RI, MS |
| 23.683 | 13 | Pinocarvone | 0.29 | 1164 | 1164 A | RI, MS |
| 25.032 | 14 | Terpinen-4-ol | 7.39 | 1183 | 1177B | RI, MS |
| 26.745 | 15 | Verbenone | 0.56 | 1207 | 1206 B | RI, MS |
| 29.015 | 16 | Unknown | 1.10 | 1243 | - | - |
| 35.372 | 17 | Piperitenone | 0.78 | 1339 | 1341 B | RI, MS |
| 39.371 | 18 | 3,5-Heptadienal, 2-ethylidene-6-methyl- | 5.71 | 1400 | 1395 Wb | RI |
| 40.828 | 19 | Unknown | 3.75 | 1425 | - | - |
| 49.798 | 20 | Caryophyllene oxide | 0.45 | 1576 | 1580 B | RI, MS |
| Monoterpene hydrocarbons: | 8.15 | |||||
| Oxygenated monoterpenes: | 76.49 | |||||
| Total monoterpenoids: | 84.64 | |||||
| Sesquiterpene hydrocarbons: | 0.00 | |||||
| Oxygenated sesquiterpenes: | 0.45 | |||||
| Total sesquiterpenoids: | 0.45 | |||||
| Total identified: | 85.09 | |||||
| Unidentified: | 9.44 | |||||
| Total | 94.53 | |||||
| Sample | Micro-Organisms | ||
|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | |
| O. suffruticosum | 12.8 | 12.8 | 6.4 |
| Ampicillin | <0.2 | <0.2 | R * |
| Sample | DPPH * | ABTS * | FRAP * | ORAC * | |||
|---|---|---|---|---|---|---|---|
| mg/mL | % RSA6 min ± SD | % RSA6 min ± SD | TEAC (μmol TE/L ± SD) | mg/mL | FRAP (μmol AAE/L ± SD) | ORAC (μmol TE/L ± SD) | |
| O. suffruticosum | 2 | 10.03 ± 1.02 | 87.17 ± 0.76 | 9431.2 ± 81.5 | 2 | −505.8 ± 80.8 | 6701.8 ± 57.2 |
| 1 | 8.38 ± 0.24 | 81.13 ± 0.51 | 8784.6 ± 54.5 | ||||
| 0.5 | 7.06 ± 0.20 | 71.46 ± 0.04 | 7750.1 ± 4.5 | ||||
| Trolox® | 2 | 94.94 ± 0.02 | – | – | – | – | – |
| 1 | 94.78 ± 0.06 | ||||||
| 0.5 | 94.45 ± 0.04 | ||||||
| Gallic acid | 2 | – | 97.97 ± 0.13 | 605,840 ± 27,811.3 | 2 | 635,500 ± 4070.9 | – |
| 1 | 97.96 ± 0.16 | 355,740 ± 7127.6 | |||||
| 0.5 | 98.05 ± 0.03 | 195,220 ± 6241.5 | |||||
| EGCG ** | – | – | – | – | 2 | – | 26,904 ± 328.2 |
| Tyrosinase Inhibition (%) | ||
|---|---|---|
| Samples | at 200 μg/mL | at 50 μg/mL |
| O. suffruticosum | 61.46 ± 11.0 | 26.14 ± 3.74 |
| Kojic acid | 96.24 ± 3.62 | 98.34 ± 0.80 |
| Wavelength (nm) | EE(λ) x I(λ) ** Employed | Absorbance * |
|---|---|---|
| 290 | 0.0150 | 0.2844 ± 0.0075 |
| 295 | 0.0817 | 0.2759 ± 0.0023 |
| 300 | 0.2874 | 0.2647 ± 0.0065 |
| 305 | 0.3278 | 0.2340 ± 0.0053 |
| 310 | 0.1864 | 0.1919 ± 0.0049 |
| 315 | 0.0837 | 0.1501 ± 0.0038 |
| 320 | 0.0180 | 0.1115 ± 0.0030 |
| Calculated SPF | 2.299 | |
| Wavelength (nm) | EE X I (Normalized) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0837 |
| 320 | 0.0180 |
| Total | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adewinogo, S.O.; Sharma, R.; Africa, C.W.J.; Marnewick, J.L.; Hussein, A.A. Chemical Composition and Cosmeceutical Potential of the Essential Oil of Oncosiphon suffruticosum (L.) Källersjö. Plants 2021, 10, 1315. https://doi.org/10.3390/plants10071315
Adewinogo SO, Sharma R, Africa CWJ, Marnewick JL, Hussein AA. Chemical Composition and Cosmeceutical Potential of the Essential Oil of Oncosiphon suffruticosum (L.) Källersjö. Plants. 2021; 10(7):1315. https://doi.org/10.3390/plants10071315
Chicago/Turabian StyleAdewinogo, Selena O., Rajan Sharma, Charlene W. J. Africa, Jeanine L. Marnewick, and Ahmed A. Hussein. 2021. "Chemical Composition and Cosmeceutical Potential of the Essential Oil of Oncosiphon suffruticosum (L.) Källersjö" Plants 10, no. 7: 1315. https://doi.org/10.3390/plants10071315
APA StyleAdewinogo, S. O., Sharma, R., Africa, C. W. J., Marnewick, J. L., & Hussein, A. A. (2021). Chemical Composition and Cosmeceutical Potential of the Essential Oil of Oncosiphon suffruticosum (L.) Källersjö. Plants, 10(7), 1315. https://doi.org/10.3390/plants10071315