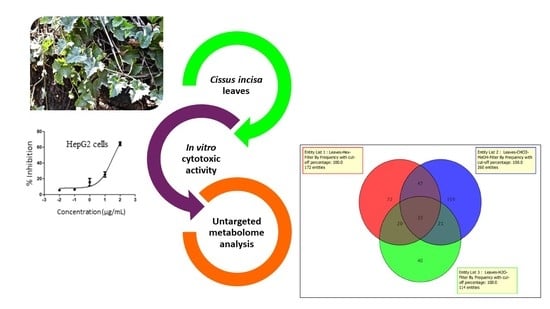
Metabolomic Profile and Cytotoxic Activity of Cissus incisa Leaves Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolomic Profile Analysis of the Extracts
2.2. Cytotoxic Activity
2.3. Metabolomics Pathway Analysis
2.4. Correspondence between Metabolomic Profiling and Cytotoxic Activity
2.5. Network Pharmacology (NP)
3. Materials and Methods
3.1. Vegetal Material and Extracts Preparation
3.2. UHPLC-QTOF-MS/MS Analysis
3.3. Data Processing and Metabolic Pathway Analysis
3.4. Cytotoxic Activity
3.4.1. Cell Lines
3.4.2. IC50 Determination
3.4.3. Selectivity Index
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Kinghorn, A.D. Development of Anticancer Agents from Plant-Derived Sesquiterpene Lactones. Curr. Med. Chem. 2016, 23, 2397–2420. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. Anal. 2020, 31, 204–214. [Google Scholar] [CrossRef]
- Commisso, M.; Strazzer, P.; Toffali, K.; Stocchero, M.; Guzzo, F. Untargeted metabolomics: An emerging approach to determine the composition of herbal pro-ducts. Comput. Struct. Biotechnol. J. 2013, 4, e201301007. [Google Scholar] [CrossRef] [Green Version]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Network Pharmacology. Innov. Approaches Drug Discov. 2017, 127–164. [Google Scholar] [CrossRef]
- PlantDataBase. Cissus Incisa. Available online: https://www.wildflower.org/plants/result.php?id_plant=citr2 (accessed on 12 August 2019).
- Alvarado Vázquez, M.A.; Rocha Estrada, A.; Moreno Limón, S. De La Lechuguilla a Las Biopelículas Vegetales: Las Plantas Útiles de Nuevo León; Universidad Autónoma de Nuevo León: Monterrey, Mexico, 2010. [Google Scholar]
- Nocedo-Mena, D.; Garza-González, E.; González-Ferrara, M.; Camacho-Corona, M.D.R. Antibacterial Activity of Cissus incisa Extracts against Multidrug- Resistant Bacteria. Curr. Top. Med. Chem. 2020, 20, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Nocedo-Mena, D.; Rivas-Galindo, V.M.; Navarro, P.; Garza-González, E.; González-Maya, L.; Ríos, M.Y.; García, A.; Ávalos-Alanís, F.G.; Rodríguez-Rodríguez, J.; Camacho-Corona, M.D.R. Antibacterial and cytotoxic activities of new sphingolipids and other constituents isolated from Cissus incisa leaves. Heliyon 2020, 6, e04671. [Google Scholar] [CrossRef] [PubMed]
- Nocedo-Mena, D.; Arrasate, S.; Garza-González, E.; Rivas-Galindo, V.M.; Romo-Mancillas, A.; Munteanu, C.R.; Sotomayor, N.; Lete, E.; Barbolla, I.; Martín, C.A.; et al. Molecular docking, SAR analysis and biophysical approaches in the study of the antibacterial activity of ceramides isolated from Cissus incisa. Bioorganic Chem. 2021, 109, 104745. [Google Scholar] [CrossRef] [PubMed]
- Mendez, F.; Garza-González, E.; Ríos, M.Y.; Ramírez-Cisneros, M.Á.; Alvarez, L.; González-Maya, L.; Sánchez-Carranza, J.N.; Camacho-Corona, M.D.R.; González, G. Metabolic profile and evaluation of biological activities of extracts from the stems of cissus trifoliata. Int. J. Mol. Sci. 2020, 21, 930. [Google Scholar] [CrossRef] [Green Version]
- Blanco, A.; Blanco, G. Lipids. In Medical Biochemistry; Academic Press: London, UK, 2017; pp. 99–119. [Google Scholar]
- Bournonville, C.G.; Filippone, M.P.; Peto, P.D.L.; Ángeles, D.; Trejo, M.F.; Couto, A.S.; de Marchese, A.M.; Ricci, J.C.D.; Welin, B.; Castagnaro, A.P. Strawberry fatty acyl glycosides enhance disease protection, have antibiotic activity and stimulate plant growth. Sci. Rep. 2020, 10, 8196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Anandan, A.; Jegadeesan, M. GC-MS Analysis of bioactive components on aerial parts of Cissus quadrangularis LT. Der. Chem. Sin. 2012, 3, 1009–1013. [Google Scholar]
- Chipiti, T.; Ibrahim, M.A.; Koorbanally, N.A.; Islam, S. In vitro antioxidant activity and GC-MS analysis of the ethanol and aqueous extracts of Cissus cornifolia (Baker) Splanch (Vitaceae) parts. Acta Pol. Pharm. Drug Res. 2015, 72, 119–127. [Google Scholar]
- Suffness, M.; Pezzuto, J. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Opoku, A.R.; Geheeb-Keller, M.; Lin, J.; Terblanche, S.E.; Hutchings, A.; Chuturgoon, A.; Pillay, D. Preliminary screening of some traditional Zulu medicinal plants for antineoplastic activities versus the HepG2 cell line. Phytother. Res. 2000, 14, 534–537. [Google Scholar] [CrossRef]
- Sahli, R.; Rivière, C.; Dufloer, C.; Beaufay, C.; Neut, C.; Bero, J.; Hennebelle, T.; Roumy, V.; Ksouri, R.; Leclercq, J.; et al. Antiproliferative and Antibacterial Activities of Cirsium scabrum from Tunisia. Evid. Based Complement. Altern. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sáenz, M.T.; García, M.D.; Quílez, A.; Ahumada, M.C. Cytotoxic activity of Agave intermixta L. (Agavaceae) and Cissus sicyoides L. (Vitaceae). Phytother. Res. 2000, 14, 552–554. [Google Scholar] [CrossRef]
- Toll, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2014, 123, 127–141. [Google Scholar]
- Lai, T.; Su, C.; Kuo, W.; Yeh, Y.; Kuo, W.; Tsai, F.; Tsai, C.; Weng, Y. β-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol. Rep. 2011, 26, 415–422. [Google Scholar] [PubMed]
- Shen, S.; Li, W.; Ouyang, M.-A.; Wang, J. Structure-activity relationship of Triterpenes and derived Glycosides against cancer cells and mechanism of apoptosis induction. Nat. Prod. Res. 2017, 32, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-D.; Yan, Y.; Jiang, C.-X.; Liang, J.-J.; Li, H.-F.; Wu, Q.-X.; Zhu, Y. Sesquiterpenes and diterpenes with cytotoxic activities from the aerial parts of Carpesium humile. Fitoterapia 2018, 128, 50–56. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lee, H.-Z.; Chen, H.-C.; Wen, C.-L.; Kuo, Y.-H.; Wang, G.-J. Anti-Inflammatory Components from the Root of Solanum erianthum. Int. J. Mol. Sci. 2013, 14, 12581–12592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Lee, H.J.; Jung, J.H.; Kim, Y.H.; Jung, D.B.; Sohn, E.J.; Lee, J.H.; Woo, H.J.; Baek, N.I.; Kim, Y.C.; et al. Activation of Caspase-9/3 and Inhibition of Epithelial Mesenchymal Transition Are Cri-tically Involved in Antitumor Effect of Phytol in Hepatocellular Carcinoma Cells. Phytother. Res. 2015, 29, 1026–1031. [Google Scholar] [CrossRef]
- Feng, H.; Zang, L.; Zhao, Z.-X.; Kan, Q.-C. Cucurbitacin-E Inhibits Multiple Cancer Cells Proliferation Through Attenuation of Wnt/β-Catenin Signaling. Cancer Biother. Radiopharm. 2014, 29, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Bar, F.M.A.; Abbas, G.M.; Gohar, A.A.; Lahloub, M.-F.I. Antiproliferative activity of stilbene derivatives and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2020, 34, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.-L.; Yu, F.-L.; Chang, C.-D.; Liao, M.-H.; Wu, H.-Y.; Lin, P.-Y. Suppression of AMF/PGI-mediated tumorigenic activities by ursolic acid in cultured hepatoma cells and in a mouse model. Mol. Carcinog. 2012, 52, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Huang, C.-Y.; Mong, M.-C.; Chan, C.-Y.; Yin, M.-C. Antiangiogenic Potential of Three Triterpenic Acids in Human Liver Cancer Cells. J. Agric. Food Chem. 2011, 59, 755–762. [Google Scholar] [CrossRef]
- Yan, S.-L.; Huang, C.-Y.; Wu, S.-T.; Yin, M.-C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. Vitr. 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Cui, H.; Han, F.; Zhang, L.; Wang, L.; Kumar, M. Gamma linolenic acid regulates PHD2 mediated hypoxia and mitochondrial apoptosis in DEN induced hepatocellular carcinoma. Drug Des. Devel. Ther. 2018, 12, 4241–4252. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.J.; Xu, M.; Wang, D.; Zhu, H.T.; Yang, C.R.; Wang, Y.F.; Li, Y.; Zhang, Y.J. Cytotoxic bisbenzylisoquinoline alkaloids from Stephania epigaea. J. Nat. Prod. 2013, 76, 926–932. [Google Scholar] [CrossRef]
- Habib, N.A.; Wood, C.B.; Apostolov, K.; Barker, W.; Hershman, M.J.; Aslam, M.; Heinemann, D.; Fermor, B.; Williamson, R.C.; Jenkins, W.E. Stearic acid and carcinogenesis. Br. J. Cancer 1987, 56, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Suo, M.-R.; Tian, Z.; Yang, J.-S.; Lu, Y.; Wu, L.; Li, W. Diterpenes from Helianthus annuus and their cytotoxicity in vitro. Yao Xue Xue Bao Acta Pharm. Sin. 2007, 42, 166–170. [Google Scholar]
- Khandanlou, R.; Murthy, V.; Wang, H. Gold nanoparticle-assisted enhancement in bioactive properties of Australian native plant extracts, Tasmannia lanceolata and Backhousia citriodora. Mater. Sci. Eng. C 2020, 112, 110922. [Google Scholar] [CrossRef] [PubMed]
- Ateba, S.B.; Mvondo, M.A.; Djiogue, S.; Zingué, S.; Krenn, L.; Njamen, D. A Pharmacological Overview of Alpinumisoflavone, a Natural Prenylated Isoflavonoid. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, X.; Lu, H.; Wen, P.; Qi, X.; Chen, S.; Wu, L.; Li, C.; Xu, A.; Zhao, G. N-(3-oxo-acyl) homoserine lactone induced germ cell apoptosis and suppressed the over-activated RAS/MAPK tumorigenesis via mitochondrial-dependent ROS in C. elegans. Apoptosis 2018, 23, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Mitome, H.; Shirato, N.; Hoshino, A.; Miyaoka, H.; Yamada, Y.; Van Soest, R.W. New polyhydroxylated sterols stylisterols A–C and a novel 5,19-cyclosterol hatomasterol from the Okinawan marine sponge Stylissa sp. Steroids 2005, 70, 63–70. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Diterpene Glycosides from the Seeds of Pharbitis nil. J. Nat. Prod. 2009, 72, 1121–1127. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Noda, Y.; Nakatani, N. Structure of constituents isolated from the bark of Cassipourea malosana and their cy-totoxicity against a human ovarian cell line. J. Nat. Med. 2019, 73, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Lin, Y.-J.; Lee, S.-J.; Yang, C.-W.; Lin, W.-Y.; Tsai, I.-L.; Chen, I.-S. Cytotoxic alkyl benzoquinones and alkyl phenols from Ardisia virens. Phytochemistry 2009, 70, 2064–2071. [Google Scholar] [CrossRef]
- Zhou, D.; Jiang, C.; Fu, C.; Chang, P.; Yang, B.; Wu, J.; Zhao, X.; Ma, S. Antiproliferative effect of 2-Hydroxy-6-tridecylbenzoic acid from ginkgo biloba sarcotestas through the aryl hydrocarbon receptor pathway in triple-negative breast cancer cells. Nat. Prod. Res. 2018, 34, 893–897. [Google Scholar] [CrossRef]
- Duarte, N.; Ramalhete, C.; Varga, A.; Molnár, J.; Ferreira, M.J.U. Multidrug resistance modulation and apoptosis induction of cancer cells by terpenic compounds isolated from Euphorbia species. Anticancer. Res. 2009, 29, 4467–4472. [Google Scholar]
- Zhi-Yong, J.; Xi-Shan, B. Cytotoxic flavanes from Uraria clarkei. J. Asian Nat. Prod. Res. 2013, 15, 979–984. [Google Scholar]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Suh, N. Cancer Prevention by Different Forms of Tocopherols. Top. Curr. Chem. 2012, 329, 21–33. [Google Scholar] [CrossRef]
- Gutierrez-Pajares, J.L.; Ben Hassen, C.; Oger, C.; Galano, J.-M.; Durand, T.; Frank, P.G. Oxidized Products of α-Linolenic Acid Negatively Regulate Cellular Survival and Motility of Breast Cancer Cells. Biomolecules 2019, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.-L.; Xiong, J.; Osman, E.E.A.; Huang, T.; Yang, G.-X.; Hu, J.-F. LC-MS guided isolation of sinodamines A and B: Chimonanthine-type alkaloids from the endangered ornamental plant Sinocalycanthus chinensis. Phytochemistry 2018, 151, 61–68. [Google Scholar] [CrossRef]
- Deyou, T.; Gumula, I.; Pang, F.; Gruhonjic, A.; Mumo, M.; Holleran, J.; Duffy, S.; Fitzpatrick, P.A.; Heydenreich, M.; Landberg, G.; et al. Rotenoids, Flavonoids, and Chalcones from the Root Bark of Millettia usaramensis. J. Nat. Prod. 2015, 78, 2932–2939. [Google Scholar] [CrossRef]
- Wang, H.-M.; Zhang, L.; Liu, J.; Yang, Z.-L.; Zhao, H.-Y.; Yang, Y.; Shen, D.; Lu, K.; Fan, Z.-C.; Yao, Q.-W.; et al. Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs. Eur. J. Med. Chem. 2015, 92, 439–448. [Google Scholar] [CrossRef]
- Li, Z.; Tran, V.H.; Duke, R.K.; Ng, M.C.; Yang, D.; Duke, C.C. Synthesis and biological activity of hydroxylated derivatives of linoleic acid and conjugated linoleic acids. Chem. Phys. Lipids 2009, 158, 39–45. [Google Scholar] [CrossRef]
- Rogovskii, V.; Popov, S.; Sturov, N.; Shimanovskii, N. The Possibility of Preventive and Therapeutic Use of Green Tea Ca-techins in Prostate Cancer. Anticancer Agents Med. Chem. 2019, 19, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 20 March 2021).
- Basu, A.; Kousuke Saito, M.; Ratna, B.K. Stellate cell apoptosis by a soluble mediator from immortalized human hepatocytes. Apoptosis 2006, 11, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]





| Identified Metabolites | Molecular Formula | Accurate Mass | Metabolite Class | Related Pathway |
|---|---|---|---|---|
| α-Tocopherolquinone * | C29H50O3 | 446.3760 | Diterpenoid | |
| Alpinumisoflavone dimethyl ether * | C22H20O5 | 364.1311 | Flavonoid | |
| 7,9,13,17-tetramethyl-7S,14S-dihydroxy-2E,4E,8E,10E,12E,16-octadecahexaenoic acid * | C22H32O4 | 360.2301 | Fatty acid derivative | |
| 7-oxo-β-Sitosterol | C29H48O2 | 428.3654 | Sterol | |
| Heneicosan-2-one * | C21H42O | 310.3236 | Fatty Acyl | |
| Gancaonin R * | C24H30O4 | 382.2144 | Stilbene | |
| 1-Monoacylglycerol | C21H36O4 | 352.2614 | Acyl glycerol | |
| 14-O-(α-L-rhamnopyranosyl)-7S,14R-dihydroxy-7,9,13,17-tetramethyl-2E,4E,8E,10E,12E,16E-octadecahexaenoic acid | C28H42O8 | 506.2880 | Fatty acid glycoside | |
| Calycanthidine * | C23H28N4 | 360.2314 | Alkaloid | |
| 1,2,6α,6β,9,9,12α-Heptamethyl-10-[(3,4,5-trihydroxyoxan-2-yl)oxy]-1,2,3,4,4α,5,6,6α,6β,7,8,8α,9,10,11,12,12α,12β,13,14β-icosahydropicene-4α-carboxylate | C35H56O7 | 588.4026 | Terpenoid | |
| 2-Heptadecylfuran * | C21H38O | 306.2923 | Heteroaromatic compound | |
| 5-Methoxy-3-(2R-acetoxy-pentadecyl)-1,4-benzoquinone | C24H38O5 | 406.2719 | Quinone | |
| Phytol * | C20H40O | 296.3079 | Diterpenoid | |
| Oxyacanthine * | C37H40N2O6 | 608.2886 | Lignan | |
| Yucalexin B16 * | C20H28O2 | 300.2089 | Diterpenoid | |
| Campesteryl p-coumarate | C37H54O3 | 546.4072 | Steroid ester | |
| 1-dodecanoyl-glycero-3-phospho-(1′-sn-glycerol) | C18H37O9P | 428.2175 | Glycerophospholipid | |
| 10,13-Epoxy-11-methyloctadeca-10,12-dienoic acid * | C19H32O3 | 308.2351 | Fatty Acyl derivative | |
| Spheroidenone | C41H58O2 | 582.4437 | Carotene derivative | |
| 2-Monopalmitoylglycerol | C19H38O4 | 330.2771 | Monoglyceride | |
| Doristerol | C27H46O | 386.3549 | Sterol | |
| (12S,15S)-15-O-demethyl-10,29-dideoxy-11,12-dihydro-striatin C | C25H38O6 | 434.2668 | Terpene | |
| δ-Tocopherol * | C27H46O2 | 402.3498 | Prenol lipid | Ubiquinone and other terpenoid-quinone biosynthesis |
| 16β-16-Hydroxy-3-oxo-1,12-oleanadien-28-oic acid | C30H44O4 | 468.3240 | Triterpene | |
| (3E)-4-(2,3-dihydroxy-2,5,5,8α-tetramethyl-decahydronaphthalen-1-yl)but-3-en-2-one * | C18H30O3 | 294.2195 | Sesquiterpenoid | |
| 3β,18β-3-Methoxy-11-oxo-12-oleanen-30-oic acid * | C31H48O5 | 484.7104 | Triterpenoid | |
| (1-cyano-2-methylprop-2-en-1-yl) 9Z,12Z-octadecadienoate | C23H37NO2 | 359.2824 | Fatty Acyl | |
| Fragarin | C21H21O10 | 434.1207 | Flavonoid | |
| Flavoxate | C24H25NO4 | 391.1784 | Flavonoid | |
| β-Citraurinene | C30H42O | 418.3236 | Triterpenoid | |
| N-(3-hydroxy-dodecanoyl)-homoserine lactone * | C16H29NO4 | 299.2097 | Fatty Acyl | |
| 1-(11Z,14Z-eicosadienoyl)-glycero-3-phosphate | C23H43O7P | 462.2746 | Glycerophospholipid | Glycerophospholipid metabolism |
| Cavipetin D | C25H38O5 | 418.2719 | Diterpenoid | |
| 10-Methoxyheptadec-1-en-4,6-diyne-3,9-diol * | C18H28O3 | 292.2038 | Fatty Acyl | |
| 1-pentadecanoyl-2-arachidonoyl-sn-glycero-3-phosphate | C38H67O8P | 682.4574 | Glycerophospholipid | |
| Diisobutyl phthalate | C16H22O4 | 278.1516 | Pollutant | |
| Lucidone A * | C24H34O5 | 402.2406 | Sesquiterpenoid | |
| 1-(9Z,12Z-octadecadienoyl)-rac-glycerol | C21H38O4 | 354.2770 | Glycerolipid | |
| (3S,5R,6S,7E,9x)-7-Megastigmene-3,6,9-triol9-glucoside | C19H34O8 | 390.2253 | Fatty acyl glycosides | |
| 5,7,4′-Trimethoxyflavan | C18H20O4 | 300.1362 | Flavonoid | |
| all-trans-Heptaprenyl diphosphate * | C35H60O7P2 | 654.3814 | Prenol lipid | |
| 1-(1Z-octadecenyl)-2-(5Z,8Z,11Z,14Z,17Z-eicosapentaenoyl)-glycero-3-phospho-(1′-sn-glycerol) | C44H77O9P | 780.5305 | Glycerophospholipid | |
| Heliotrine * | C16H27NO5 | 313.1889 | Member of pyrrolizines | |
| (all-E)-6′-Apo-y-caroten-6′-al | C32H42O | 442.3272 | Prenol lipid | |
| 1-dodecanoyl-sn-glycero-3-phosphocholine | C20H42NO7P | 439.2699 | Glycerophospholipid | |
| 1-(9Z-hexadecenoyl)-2-(11Z-eicosenoyl)-glycero-3-phosphoserine | C42H78NO10P | 787.5363 | Glycerophospholipid | |
| Stylisterol B | C28H46O4 | 446.3396 | Sterol Lipid | |
| Stylisterol A * | C28H46O3 | 430.3447 | Sterol Lipid | |
| Cucurbitacin E * | C32H44O8 | 556.3036 | Triterpenoid | |
| (22E,24R)-Stigmasta-4,22-diene-3,6-dione | C29H44O2 | 424.3341 | Lipid | |
| Junceic acid * | C21H30O3 | 330.2195 | Prenol lipid | |
| 2-Hydroxy-6-tridecylbenzoic acid * | C20H32O3 | 320.2351 | phenolic compound | |
| 1-docosanoyl-glycero-3-phospho-(1′-sn-glycerol) | C28H57O9P | 568.3740 | Glycerophospholipid | |
| Gibberellin A12 aldehyde * | C20H28O3 | 316.2038 | Prenol lipid | Diterpenoid biosynthesis |
| Matricin * | C17H22O5 | 306.1467 | Prenol lipid | |
| 19-α-19-hydroxy-3,11-dioxo-12-ursen-28-oic acid | C30H44O5 | 484.3188 | Triterpenoid | |
| α-Amyrin acetate * | C32H52O2 | 468.3967 | Triterpenoid | |
| (5α,25R)-Spirostan-3,6-dione | C27H40O4 | 428.2926 | Sterol | |
| Ent-9-L1-phytoP * | C18H28O4 | 308.1988 | Fatty Acyl | |
| γ-Linolenic Acid * | C18H30O2 | 278.2246 | Fatty acid | Biosynthesis of unsaturated fatty acids |
| Crispane | C20H32O3 | 320.2351 | Terpene | |
| Austroinulin * | C20H34O3 | 322.2508 | Diterpenoid | |
| Presqualene diphosphate * | C30H52O7P2 | 586.3188 | Terpenoid | Sesquiterpenoid and triterpenoid biosynthesis; Steroid biosynthesis |
| (−)-Folicanthine * | C24H30N4 | 374.2470 | Indoles derivative | |
| 1-Octadecanoyl-2-docosanoyl-sn-glycero-3-phosphate | C43H85O8P | 760.5982 | Glycerophospholipid | |
| Ursolic acid | C30H48O3 | 456.3603 | Triterpenoid | |
| Amabiline * | C15H25NO4 | 283.1784 | Carboxylic ester | |
| (−)-Epicatechin 3′-O-sulfate | C15H14O9S | 370.0358 | Flavonoid | |
| Stearic acid * | C18H36O2 | 284.2715 | Fatty acid | Biosynthesis of unsaturated fatty acids |
| Grandifloric acid * | C20H30O3 | 318.2194 | Terpene | |
| 1,2-Dihexadecanoylphosphatidylglycerol phosphate | C38H76O13P2 | 802.4761 | Glycerophospholipid | |
| Yucalexin B5 * | C20H26O3 | 314.1881 | Terpene | |
| 6-Deoxohomodolichosterone * | C29H50O4 | 462.3709 | Sterol Lipid | |
| 7′,8′-Dihydro-8′-hydroxycitraniaxanthin * | C33H44O3 | 488.3290 | Triterpenoid | |
| 5,6-Epoxy-5,6-dihydro-10′-apo-β,γ-carotene-3,10′-diol * | C27H38O3 | 410.2820 | Carotenoid | |
| N-tetradecanoyl glutamine * | C19H36N2O4 | 356.2675 | Fatty Acyl | |
| Stigmastane-3,6-dione * | C29H48O2 | 428.3654 | Sterol Lipid | |
| 2-Stearyl citric acid * | C24H44O7 | 444.3087 | Tricarboxylic acid | |
| 1-(4Z,7Z,10Z,13Z,16Z,19Z-Docosahexaenoyl)-2-(13Z-docosenoyl)-sn-glycero-3-phosphocholine * | C52H90NO8P | 887.6404 | Glycerophospholipid | |
| 17-Phenyl heptadecanoic acid * | C23H38O2 | 346.2872 | Fatty Acyl |
| Cell Lines | Hexane Extract | CHCl3/MeOH Extract | Aqueous Extract | Paclitaxel | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | SI | IC50 (µg/mL) | SI | IC50 (µg/mL) | SI | IC50 (µg/mL) | SI | |
| HepG2 | 30 ± 6 | 1.5 | 39 ± 3 | 2.21 | >100 | ND | 64 × 10−3 | 1.24 |
| Hep3B | 27 ± 3 | 1.66 | 31 ± 2 | 2.77 | >100 | ND | 33 × 10−3 | 2.41 |
| HeLa | 40 ± 2 | ND | 61 ± 4 | ND | >100 | ND | 4.78 × 10−3 | ND |
| A549 | 52 ± 2 | ND | 77 ± 6 | ND | >100 | ND | 5.12 × 10−3 | ND |
| PC3 | 76 ± 5 | ND | 57 ± 4 | ND | >100 | ND | 10.2 × 10−3 | ND |
| MCF7 | 74 ± 6 | ND | 50.7 ± 6 | ND | >100 | ND | 4.27 × 10−3 | ND |
| IHH | 45 ± 3 | 86 ± 5 | >100 | 79.4 × 10−3 | ||||
| No. | Pathway Name | Total * | Expected | Hits * | Raw p * | Holm p * | FDR p * | Impact * |
|---|---|---|---|---|---|---|---|---|
| 1 | Biosynthesis of unsaturated fatty acids | 22 | 0.21 | 2 | 1.72 × 10−2 | 1.00 | 1.00 | 0.00 |
| 2 | Linoleic acid metabolism | 4 | 0.04 | 1 | 3.73 × 10−2 | 1.00 | 1.00 | 0.00 |
| 3 | Sesquiterpenoid and triterpenoid biosynthesis | 24 | 0.23 | 1 | 2.05 × 10−1 | 1.00 | 1.00 | 0.20374 |
| 4 | alpha-Linolenic acid metabolism | 28 | 0.26 | 1 | 2.35 × 10−1 | 1.00 | 1.00 | 0.00 |
| 5 | Diterpenoid biosynthesis | 28 | 0.26 | 1 | 2.35 × 10−1 | 1.00 | 1.00 | 0.07625 |
| 6 | Glycerophospholipid metabolism | 37 | 0.35 | 1 | 2.99 × 10−1 | 1.00 | 1.00 | 0.07614 |
| 7 | Ubiquinone and other terpenoid-quinone biosynthesis | 38 | 0.36 | 1 | 3.06 × 10−1 | 1.00 | 1.00 | 0.02227 |
| 8 | Steroid biosynthesis | 45 | 0.42 | 1 | 3.52 × 10−1 | 1.00 | 1.00 | 0.02644 |
| 9 | Fatty acid biosynthesis | 56 | 0.56 | 1 | 4.18 × 10−1 | 1.00 | 1.00 | 0.00 |
| Metabolites | Molecular Formula | Accurate Mass | Up-Regulation [Hexane Extract] | Up-Regulation [CHCl3/MeOH Extract] | Biological Activity/ References |
|---|---|---|---|---|---|
| HeLa, A549 and/or related cell lines | |||||
| α-Tocopherolquinone | C29H50O3 | 446.3760 | Yes | - | [23] |
| Alpinumisoflavone dimethyl ether | C22H20O5 | 364.1311 | Yes | - | H2108 (IC50 = 33.5 µM); H1299 (IC50 = 38.8 µM) [36] |
| 7,9,13,17-tetramethyl-7S,14S-dihydroxy-2E,4E,8E,10E,12E,16-octadecahexaenoic acid | C22H32O4 | 360.2301 | Yes | - | |
| Heneicosan-2-one | C21H42O | 310.3236 | Yes | - | |
| Gancaonin R | C24H30O4 | 382.2144 | Yes | - | |
| Calycanthidine | C23H28N4 | 360.2314 | Yes | - | |
| 2-Heptadecylfuran | C21H38O | 306.2923 | Yes | - | |
| Phytol | C20H40O | 296.3079 | Yes | - | Hela (IC50 = 15.51 ± 0.76 µM); A549 (IC50 = 56.98 ± 2.68 µM) [45] |
| Yucalexin B16 | C20H28O2 | 300.2089 | Yes | - | |
| δ-Tocopherol | C27H46O2 | 402.3498 | Yes | - | [46] |
| (3E)-4-(2,3-dihydroxy-2,5,5,8α-tetramethyl-decahydronaphthalen-1-yl)but-3-en-2-one | C18H30O3 | 294.2195 | Yes | - | |
| 3β,18β-3-Methoxy-11-oxo-12-oleanen-30-oic acid | C31H48O5 | 484.7104 | Yes | - | |
| N-(3-hydroxy-dodecanoyl)-homoserine lactone | C16H29NO4 | 299.2097 | Yes | - | [37] |
| 10-methoxyheptadec-1-en-4,6-diyne-3,9-diol | C18H28O3 | 292.2038 | Yes | - | |
| Lucidone A | C24H34O5 | 402.2406 | Yes | - | |
| Stylisterol A | C28H46O3 | 430.3447 | Yes | - | HeLa (IC50 = 14.1 µM) [38] |
| Cucurbitacin E | C32H44O8 | 556.3036 | Yes | - | [26] |
| gibberellin A12 aldehyde | C20H28O3 | 316.2038 | Yes | - | [39] |
| α-Amyrin acetate | C32H52O2 | 468.3967 | Yes | - | [27] |
| Ent-9-L1-phytoP | C18H28O4 | 308.1988 | Yes | - | [47] |
| γ-Linolenic Acid | C18H30O2 | 278.2246 | Yes | - | [31] |
| (−)-Folicanthine | C24H30N4 | 374.2470 | Yes | - | A549 (IC50 = 7.76 µM) [48] |
| Amabiline | C15H25NO4 | 283.1784 | Yes | - | |
| Grandifloric acid | C20H30O3 | 318.2194 | Yes | - | [34] |
| Yucalexin B5 | C20H26O3 | 314.1881 | Yes | - | |
| 7′,8′-Dihydro-8′-hydroxycitraniaxanthin | C33H44O3 | 488.3290 | Yes | - | |
| 5,6-Epoxy-5,6-dihydro-10′-apo-β,γ-carotene-3,10′-diol | C27H38O3 | 410.2820 | Yes | - | |
| N-tetradecanoyl glutamine | C19H36N2O4 | 356.2675 | Yes | - | |
| 2-Stearyl citric acid | C24H44O7 | 444.3087 | Yes | - | |
| 17-phenyl heptadecanoic acid | C23H38O2 | 346.2872 | Yes | - | |
| PC3, MCF7 and/or related cell lines | |||||
| 4′-O-Geranylisoliquiritigenin | C25H28O4 | 392.1988 | - | Yes | MDB-MB-231 (IC50 = 125.5 µM) [49] |
| 1-Monoacylglycerol | C20H34NO4 | 352.2619 | - | Yes | |
| Sanguisorbin B | C35H56O7 | 588.4026 | - | Yes | |
| Oxyacanthine | C37H40N2O6 | 608.2886 | - | Yes | [32] |
| 3-Methyl-5-pentyl-2-furannonanoic acid | C19H32O3 | 308.2351 | - | Yes | |
| 2-Monopalmitoylglycerol | C19H38O4 | 330.2771 | - | Yes | |
| 15-hydroxy-5,9-dimethyl-14-methylidenetetracyclo[11.2.1.01,10.04,9]hexadecane-5-carboxylic acid | C20H30O3 | 318.2194 | - | Yes | |
| 4-hydroxy-8-cis-sphingenine | C18H37NO3 | 315.2773 | - | Yes | |
| N-(5-aminopentyl)-N’-(5-{[4-({5-[butylidene(oxido)-lambda(5)-azanyl]pentyl}amino)-4-oxobutanoyl](hydroxy)amino}pentyl)-N-hydroxybutanediamide | C27H52N6O7 | 572.3897 | - | Yes | |
| 1,2-di-(9Z-pentadecenoyl)-sn-glycerol | C33H60O5 | 536.4441 | - | Yes | |
| 4,2′,4′-Trihydroxy-3′,5′-diprenylchalcone | C25H28O4 | 392.1988 | - | Yes | [50] |
| 5,7,4′-Trimethoxyflavan | C18H20O4 | 300.1362 | - | Yes | |
| all-trans-Heptaprenyl diphosphate | C35H60O7P2 | 654.3814 | - | Yes | |
| Heliotrine | C16H27NO5 | 313.1889 | - | Yes | |
| (22E,24R)-Stigmasta-4,22-diene-3,6-dione | C29H44O2 | 424.3341 | - | Yes | |
| Methyl 9R-hydroxy-10E,12E-octadecadienoate | C19H34O3 | 310.2508 | - | Yes | [51] |
| 1,2-di-(9Z,12Z-octadecadienoyl)-sn-glycerol | C39H68O5 | 616.5067 | - | Yes | |
| N-(1,3-dihydroxypropan-2-yl)hexadecanamide | C21H30O3 | 330.2195 | - | Yes | |
| 2-Hydroxy-6-tridecylbenzoic acid | C20H32O3 | 320.2351 | - | Yes | MDA-MB-231 (IC50 = 117.25 µM); 4T-1 (IC50 = 102.39 µM) [42] |
| β-isorenieratane | C40H72 | 552.5634 | - | Yes | |
| Matricin | C17H22O5 | 306.1467 | - | Yes | [35] |
| 9,10,13-trihydroxy-octadecanoic acid | C18H36O5 | 332.2563 | - | Yes | |
| Austroinulin | C20H34O3 | 322.2508 | - | Yes | |
| Butyl 3-O-β-D-glucopyranosyl-butanoate | C14H26O8 | 322.1628 | - | Yes | |
| Presqualene diphosphate | C30H52O7P2 | 586.3188 | - | Yes | |
| 2-O-(β-D-galactopyranosyl-(1->6)-β-D-galactopyranosyl) 2S,3R-dihydroxynonanoic acid | C21H38O14 | 514.2262 | - | Yes | |
| Catechin 3-O-rutinoside | C27H34O15 | 598.1898 | - | Yes | [52] |
| Stearic acid | C18H36O2 | 284.2715 | - | Yes | [33] |
| 1-O-(2R-methoxy-4Z-heneicosenyl)-sn-glycerol | C25H50O4 | 414.3709 | - | Yes | |
| Ammothamnidin | C25H28O5 | 408.1937 | - | Yes | |
| 6-Deoxohomodolichosterone | C29H50O4 | 462.3709 | - | Yes | |
| 1-hexadecyl-glycero-3-phospho-(1′-sn-glycerol) | C22H47O8P | 470.3009 | - | Yes | |
| 28-Glucopyranosyl-3-methyloleanolic acid | C37H60O8 | 632.4285 | - | Yes | |
| Stigmastane-3,6-dione | C29H48O2 | 428.3654 | - | Yes | |
| N-(dodecanoyl)-sphing-4-enine | C30H59NO3 | 481.4503 | - | Yes | |
| Phyllospadine | C21H21NO6 | 383.1369 | - | Yes | |
| Tricosylic acid | C23H46O2 | 354.3498 | - | Yes | |
| 1-Docosahexaenoyl-2-erucoyl-sn-glycero-3-phosphocholine | C52H90NO8P | 887.6404 | - | Yes | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocedo-Mena, D.; Ríos, M.Y.; Ramírez-Cisneros, M.Á.; González-Maya, L.; Sánchez-Carranza, J.N.; Camacho-Corona, M.d.R. Metabolomic Profile and Cytotoxic Activity of Cissus incisa Leaves Extracts. Plants 2021, 10, 1389. https://doi.org/10.3390/plants10071389
Nocedo-Mena D, Ríos MY, Ramírez-Cisneros MÁ, González-Maya L, Sánchez-Carranza JN, Camacho-Corona MdR. Metabolomic Profile and Cytotoxic Activity of Cissus incisa Leaves Extracts. Plants. 2021; 10(7):1389. https://doi.org/10.3390/plants10071389
Chicago/Turabian StyleNocedo-Mena, Deyani, María Yolanda Ríos, M. Ángeles Ramírez-Cisneros, Leticia González-Maya, Jessica N. Sánchez-Carranza, and María del Rayo Camacho-Corona. 2021. "Metabolomic Profile and Cytotoxic Activity of Cissus incisa Leaves Extracts" Plants 10, no. 7: 1389. https://doi.org/10.3390/plants10071389
APA StyleNocedo-Mena, D., Ríos, M. Y., Ramírez-Cisneros, M. Á., González-Maya, L., Sánchez-Carranza, J. N., & Camacho-Corona, M. d. R. (2021). Metabolomic Profile and Cytotoxic Activity of Cissus incisa Leaves Extracts. Plants, 10(7), 1389. https://doi.org/10.3390/plants10071389