Apple (Malus domestica Borkh.) Cultivar ‘Majda’, a Naturally Non-Browning Cultivar: An Assessment of Its Qualities
Abstract
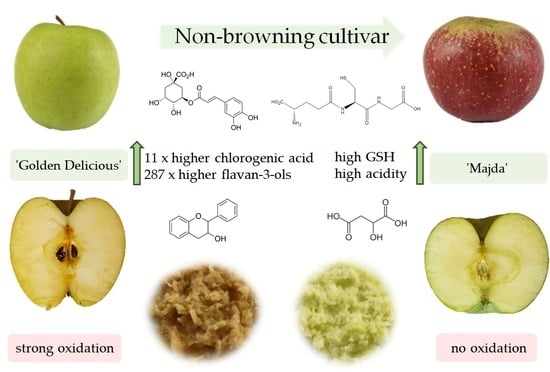
:1. Introduction
2. Results
2.1. Sugars
2.2. Organic Acids
2.3. pH
2.4. Color Change
2.5. Vitamin C, Methionine, L-cysteine, GSH and GSSG
2.6. Phenolic Content
2.7. PPO and POX
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Color Analysis
4.3. Sugar and Organic Acid Extraction and Analysis
4.4. Vitamin C Extraction and Analysis
4.5. Phenolic Content Extraction and Analysis
4.6. Glutathione (GSH, GSSH), Cystein and Methionine Extraction, and Analysis
4.7. POX and PPO Activity Determination
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Tien, C.; Vachon, C.; Mateescu, M.-A.; Lacroix, M. Milk protein coatings prevent oxidative browning of apples and potatoes. J. Food Sci. 2001, 66, 512–516. [Google Scholar] [CrossRef]
- Gacche, R.N.; Warangkar, S.C.; Ghole, V.S. Glutathione and cinnamic acid: Natural dietary components used in preventing the process of browning by inhibition of polyphenol oxidase in apple juice. J. Enzym. Inhib. Med. Chem. 2004, 19, 175–179. [Google Scholar] [CrossRef]
- Khanizadeh, S.; Groleau, Y.; Levasseur, A.; Charles, M.T.; Tsao, R.; Yang, R.; DeEll, J.; Hampson, C.; Toivonen, P.T. SJCA38R6A74 (Eden). HortScience 2006, 41, 1513–1515. [Google Scholar] [CrossRef]
- Tazawa, J.; Oshino, H.; Kon, T.; Kasai, S.; Kudo, T.; Hatsuyama, Y. Genetic characterization of flesh browning trait in apple using the non-browning cultivar ‘Aori 27’. Tree Genet. Genomes 2019, 15, 49. [Google Scholar] [CrossRef]
- Waltz, E. Nonbrowning GM apple cleared for market. Nat. Biotechnol. 2015, 33, 326–327. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, V.L.V.; Queiroz, C. Enzymatic browning. In Biochemistry of Foods; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: London, UK, 2013; Volume 3, pp. 387–418. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicolas, J. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J. Food Sci. 1992, 57, 958–962. [Google Scholar] [CrossRef]
- Demeke, T.; Morris, C.F. Molecular characterization of wheat polyphenol oxidase (PPO). Theor. Appl. Genet. 2002, 104, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, M.; Tadiello, A.; Farneti, B.; Lorenz, G.; Masuero, D.; Vrhovsek, U.; Costa, G.; Velasco, R.; Costa, F. A multidisciplinary approach providing new insight into fruit flesh browning physiology in apple (Malus × domestica Borkh). PLoS ONE 2013, 8, e78004. [Google Scholar] [CrossRef] [PubMed]
- Ambrosia, T.M. Why Do Apples Turn Brown? Available online: https://ambrosiaapples.ca/apples-turn-brown/ (accessed on 23 March 2021).
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- May, M.J.; Vernoux, T.; Leaver, C.; Van Montagu, M.; Inzé, D. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Experiment. Bot. 1998, 49, 649–667. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Foito, A.; Dobson, G.; Austin, C.; Sungurtas, J.; Su, S.; Wang, L.; Feng, C.; Li, S.; Wang, L.; et al. Glutathionyl-S-chlorogenic acid is present in fruit of Vaccinium species, potato tubers and apple juice. Food Chem. 2020, 330, 127227. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric acid disappearance and conversion to products of enzymic oxidation in grape must and wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Qeval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Awad, M.A.; Jager, A. Influences of air and controlled atmosphere storage on the concentration of potentially helathful phenolics in apples and other fruits. Postharvest Biol. Technol. 2003, 27, 53–58. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Ban, Z.; Jiang, Y. Variation in antioxidant metabolites and enzymes of ‘Red Fuji’ apple pulp and peel during cold storage. Int. J. Food Prop. 2014, 17, 1067–1080. [Google Scholar] [CrossRef]
- Davey, M.W.; Keulemans, J. Determining the potential to breeed for enhanced antioxidant status in Malus:mean inter- and intravarietal fruit vitamin C and glutathiones contents at harvest and their evolution during storage. J. Agric. Food Chem. 2004, 52, 8031–8038. [Google Scholar] [CrossRef]
- Crnko, J.; Marn, M. ‘Majda’ nova Jugoslovanska jablanova sorta za dvojno uporabo. In Zbornik Biotehniske Fakultete Univerze Edvarda Kardelja v Ljubljani: Kmetijstvo; Biotechnical Faculty: Ljubljana, Slovenia, 1986; Volume 47, pp. 49–62. [Google Scholar]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Rymenants, M.; van de Weg, E.; Auwerkerken, A.; De Wit, I.; Czech, A.; Nijland, B.; Heuven, H.; De Storme, N.; Keulemans, W. Detection of QTL for apple fruit acidity and sweetness using sensorial evaluation in multiple pedigreed full-sib families. Tree Genet. Genomes 2020, 16, 71. [Google Scholar] [CrossRef]
- Morimoto, T.; Yonemushi, K.; Ohnishi, H.; Banno, K. Genetic and physical mapping of QTLs for fruit juice browning and fruit acidity on linkage group 16 in apple. Tree Genet. Mol. Breed. 2014, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maliepaard, C.; Alston, F.H.; van Arkel, G.; Brown, L.M.; Chevreau, E.; Dunemann, F.; Evans, K.M.; Gardiner, S.; Guilford, P.; van Heusden, A.W.; et al. Aligning male and female linkage maps of apple (Malus pumila Mill.) using multi-allelic markers. Theor. Appl. Genet. 1998, 97, 60–73. [Google Scholar] [CrossRef]
- Joshi, A.P.K.; Rupasinghe, H.P.V.; Pitts, N.L.; Khanizadeh, S. Biochemical characterization of enzymatic browning in selected apple genotypes. Can. J. Plant Sci. 2007, 87, 1067–1074. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 2, 109–157. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2018, 9, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritziger, E.C.; Bauer, F.F.; du Toit, W.J. Role of glutathione in winemaking: A review. J. Agric. Food Chem. 2013, 2, 269–277. [Google Scholar]
- Boss, P.K.; Gardner, R.C.; Janssen, B.-J.; Ross, G.S. An apple polypgenol oxidase cDNA is up-regulated in wounded tissues. Plant Mol. Biol. 1995, 27, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Janovitz-Klapp, A.H.; Richard, F.C.; Goupy, M.; Nicolas, J.J. Kinetic studies on apple polyphenol oxidase. J. Agric. Food Chem. 1990, 38, 1437–1441. [Google Scholar] [CrossRef]
- Dugé de Bernonville, T.; Guyot, S.; Paulin, J.-P.; Gaucher, M.; Loufrani, L.; Henrion, D.; Derbré, S.; Guilet, D.; Richomme, P.; Dat, J.F.; et al. Dihydrochalcones: Implication in resistance to oxidative stress and bioactivities against advanced glycation end-products and vasoconstriction. Phytochemistry 2010, 71, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Yuri, A.J.; Neira, A.; Quilodran, A.; Razmilic, I.; Motomura, Y.; Torres, C.; Palomo, I. Sunburn on apples is associated with increases in phenolic compounds and antioxidant activity as a function of the cultivar and areas of the fruit. J. Food Agric. Environ. 2010, 8, 920–925. [Google Scholar]
- Łata, B.; Tomala, K. Apple Peel as a Contributor to whole fruit quantity of potentially healthful bioactive compounds. cultivar and year Implication. J Agric. Food Chem. 2007, 55, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Parameters of inner quality of the apple scab resistant and susceptible apple cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Janeš, L.; Požgan, F.; Bolta, Š.V.; Sivilotti, P.; Lisjak, K. UHPLC-MS/MS determination of varietal thiol precursors in Sauvignon Blanc grapes. Sci. Rep. 2017, 7, 13122. [Google Scholar] [CrossRef] [Green Version]
- Zupan, A.; Mikulic-Petkovsek, M.; Slantnar, A.; Stampar, F.; Veberic, R. Individual phenolic response and peroxidase activity in peel of differently sun-exposed apples in the period favorable for sunburn occurrence. J. Plant Physiol. 2014, 171, 1706–1712. [Google Scholar] [CrossRef]
- Cebulj, A.; Halbwirth, H.; Mikulic-Petkovsek, M.; Veberič, R.; Slatnar, A. The impact of scald development on phenylpropanoid metabolism based on phenol content, enzyme activity, and gene expression analysis. Hortic. Environ. Biotechnol. 2020, 61, 849–858. [Google Scholar] [CrossRef]









| Cul | Loc | T | Cul:Loc | T:Cul | T:Loc | T:Cul:Loc | |
|---|---|---|---|---|---|---|---|
| Total sugars | ** | . | *** | NS | ** | . | NS |
| Total acids | *** | NS | *** | ** | NS | ** | *** |
| pH | *** | NS | *** | NS | ** | NS | NS |
| Vitamin C | *** | ** | *** | ** | *** | * | NS |
| Methionine | *** | *** | *** | NS | *** | *** | * |
| Cysteine | NS | NS | *** | NS | *** | NS | NS |
| GSH | *** | * | *** | NS | . | ** | * |
| GSSG | ** | NS | NS | NS | NS | NS | NS |
| Hydroxycinnamic acids | *** | ** | *** | NS | NS | NS | NS |
| Dihydrochalcones | *** | NS | *** | NS | ** | NS | NS |
| Flavonols | *** | ** | . | * | NS | NS | NS |
| Flavan-3-ols | *** | NS | NS | NS | NS | NS | NS |
| PPO | * | ** | ** | NS | NS | ** | NS |
| POX | NS | NS | *** | ** | NS | NS | NS |
| Cul | T | Trt | Cul:T | Trt:Cul | Trt:T | Tr:Cul:T | |
| ΔE Δt−1 | *** | NS | *** | . | NS | *** | NS |
| Cul | Loc | Cul:Loc | |
|---|---|---|---|
| Peel | |||
| Hydroxycinnamic acids | *** | *** | NS |
| Dihydrochalcones | NS | NS | NS |
| Flavonols | NS | * | NS |
| Flavan-3-ols | *** | *** | NS |
| Leaves | |||
| Arbutin | * | ** | NS |
| Hydroxycinnamic acids | * | . | NS |
| Dihydrochalcones | *** | * | NS |
| Flavonols | *** | . | NS |
| Flavan-3-ols | *** | *** | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebulj, A.; Vanzo, A.; Hladnik, J.; Kastelec, D.; Vrhovsek, U. Apple (Malus domestica Borkh.) Cultivar ‘Majda’, a Naturally Non-Browning Cultivar: An Assessment of Its Qualities. Plants 2021, 10, 1402. https://doi.org/10.3390/plants10071402
Cebulj A, Vanzo A, Hladnik J, Kastelec D, Vrhovsek U. Apple (Malus domestica Borkh.) Cultivar ‘Majda’, a Naturally Non-Browning Cultivar: An Assessment of Its Qualities. Plants. 2021; 10(7):1402. https://doi.org/10.3390/plants10071402
Chicago/Turabian StyleCebulj, Anka, Andreja Vanzo, Joze Hladnik, Damijana Kastelec, and Urska Vrhovsek. 2021. "Apple (Malus domestica Borkh.) Cultivar ‘Majda’, a Naturally Non-Browning Cultivar: An Assessment of Its Qualities" Plants 10, no. 7: 1402. https://doi.org/10.3390/plants10071402
APA StyleCebulj, A., Vanzo, A., Hladnik, J., Kastelec, D., & Vrhovsek, U. (2021). Apple (Malus domestica Borkh.) Cultivar ‘Majda’, a Naturally Non-Browning Cultivar: An Assessment of Its Qualities. Plants, 10(7), 1402. https://doi.org/10.3390/plants10071402