Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation
Abstract
:1. Introduction
2. Results
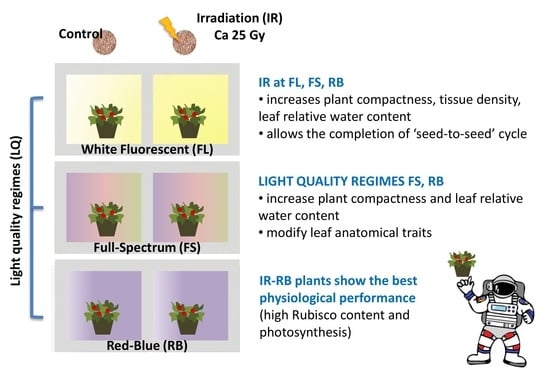
2.1. Effect of Ionizing Radiation and Light Quality on Plant Growth and Leaf Functional Traits
2.2. Effect of Ionizing Radiation and Light Quality on Photosynthetic Gas Exchanges
2.3. Effect of Ionizing Radiation and Light Quality on Biochemical Compounds, Proteins and Rubisco
2.4. Heatmap Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material, Sample Irradiation and Experimental Plan
4.2. Seed Germination, Biometrical Measurement and Leaf Functional Traits
4.3. Anatomical Analyses
4.4. Gas Exchange Measurements
4.5. Total Protein and Rubisco Amount
4.6. Leaf Biochemical Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sust. Energ. Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.G.; Hay Mele, B.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Yavari, N.; Tripathi, R.; Wu, B.S.; MacPherson, S.; Singh, J.; Lefsrud, M. The effect of light quality on plant physiology, photosynthetic, and stress response in Arabidopsis thaliana leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; De Micco, V.; De Maio, A. Growth alteration and leaf biochemical responses in P. vulgaris plants exposed to different doses of ionizing radiation. Plant Biol. 2014, 16, 194–202. [Google Scholar] [CrossRef]
- Honda, I.; Kikuchi, K.; Matsuo, S.; Fukuda, M.; Saito, H.; Ryuto, H.; Fukunishi, N.; Abe, T. Heavy-ioninduced mutants in sweet pepper isolated by M1 plant selection. Euphytica 2006, 152, 61–66. [Google Scholar] [CrossRef]
- Hou, S.; Sun, L.; Zhang, Y.; Wu, D.; Guan, L.; Gao, Q.; Li, W.; Dang, B.; Xie, H.; Zhou, L. Mutagenic effects of Brassica napus by 12C6+ ion beam. Nucl. Technol. 2008, 31, 449–454. [Google Scholar]
- Xie, Z.K.; Wang, Y.J.; Xie, H.M.; Guo, Z.H.; Wei, Z.Q. Study of mutation breeding with heavy ion irradiation on potatoes. Nucl. Phys. Rev. 2008, 25, 187–190. [Google Scholar]
- Kharkwal, M.C. A brief history of plant mutagenesis. In Plant Mutation Breeding and Biotechnology; Shu, Q.Y., Foster, B.P., Nakagawa, H., Eds.; CABI: Wallingford, UK, 2012; pp. 21–30. [Google Scholar]
- Dong, X.; Yan, X.; Li, W. Plant mutation breeding with heavy ion irradiation at IMP. J. Agric. Sci. 2016, 8, 1916–9760. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.D.; Kim, S.H.; Hwang, J.E.; Kim, Y.S.; Kang, H.S.; Kim, S.W.; Kwon, S.J.; Ryu, J.; Kim, J.B.; Kang, S.Y. Construction of mutation populations by Gamma-ray and Carbon beam irradiated in Chili pepper (Capsicum annuum L.). Hortic. Environ. Biotechnol. 2016, 57, 606–614. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Arena, C.; Vitale, E.; Hay Mele, B.; Cataletto, P.R.; Turano, M.; Simoniello, P.; De Micco, V. Suitability of Solanum lycopersicum L. ‘Microtom’ for growth in Bioregenerative Life Support Systems: Exploring the effect of high-LET ionising radiation on photosynthesis, leaf structure and fruit traits. Plant Biol. 2019, 21, 615–626. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of sparsely and densely ionizing radiation on plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Wang, G.; Chen, X.; Li, H.; Yang, H.; Wang, L.; Gao, Q.; Wang, C.; Wang, Y. Biological effects of carbon ions with medium energy on plant seeds. Radiat. Res. 1995, 141, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Shimono, K.; Inoue, M.; Tanaka, A.; Watanabe, H. Biological effects of ion beams in Nicotiana tabacum L. Radiat. Environ. Biophys. 1999, 38, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Komai, F.; Shikazono, N.; Tanaka, A. Sexual modification of female spinach seeds (Spinacia oleracea L.) by irradiation with ion particles. Plant Cell Rep. 2003, 21, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Sjahril, R.; Riadi, M.; Raffiundin, S.T.; Toriyama, K.; Abe, T.; Trisnawaty, A.R. Effect of heavy ion beam irradiation on germination of local Toraja rice seed (M1-M2) mutant generation. IOP Conf. Ser. Earth Environ. Sci. 2018, 157, 012046. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Macaeva, E.; Quintens, R. Space radiation effects on plant and mammalian cells. Acta Astronaut 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Okazaki, M.; Ezura, H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivat Micro-Tom. J. Pineal Res. 2009, 46, 338–343. [Google Scholar] [CrossRef]
- Stürtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef]
- Mei, M.; Deng, H.; Lu, Y.; Zhuang, C.; Liu, Z.; Qiu, Y.; Yang, T.C. Mutagenic effects of heavy ion radiation in plants. Adv. Space Res. 1994, 14, 363–372. [Google Scholar] [CrossRef]
- De Micco, V.; Paradiso, R.; Aronne, G.; De Pascale, S.; Quarto, M.; Arena, C. Leaf anatomy and photochemical behaviour of Solanum lycopersicum L. plants from seeds irradiated with low-LET ionizing radiation. Sci. World J. 2014, 2014, 428141. [Google Scholar] [CrossRef] [Green Version]
- Nechitailo, G.S.; Jinying, L.; Huai, X.; Yi, P.; Chongqin, T.; Min, L. Influence of long-term exposure to space flight on tomato seeds. Adv. Space Res. 2005, 36, 1329–1333. [Google Scholar] [CrossRef]
- Dougher, T.A.; Bugbee, B. Long-term blue effect on histology of lettuce and soybean leaves and stems. J. Am. Soc. Hortic. Sci. 2004, 129, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Xiaoying, L.; Shirong, G.; Taotao, C.; Zhigang, X.; Tezuka, T. Regulation of the growth and photosynthesis of cherry tomato seedlings by different light irradiations of light emitting diodes (LED). Afr. J. Biotechnol. 2012, 11, 6169–6177. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T.; Arena, C. The interplay between light quality and biostimulant application affects the antioxidant capacity and photosynthetic traits of soybean (Glycine max. L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Ryser, P.; Urbas, P. Ecological significance of leaf life span among Central European grass species. Oikos 2000, 91, 41–50. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving Spinach, Radish, and Lettuce Growth under Red Light emitting Diodes (LEDs) with Blue Light Supplementation. Hortic. Sci. 2001, 36, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Dutta Gupta, S. Impact of light-emitting diodes (LEDs) and its potential on plant growth and development in controlled-environment plant production system. Curr. Biotechnol. 2016, 5, 28–43. [Google Scholar] [CrossRef]
- Buckley, T.N.; John, G.P.; Scoffoni, C.; Sack, L. How does leaf anatomy influence water transport outside the xylem? Plant Physiol. 2015, 168, 1616–1635. [Google Scholar] [CrossRef] [Green Version]
- Amitrano, C.; Arena, C.; Rouphael, Y.; De Pascale, S.; De Micco, V. Vapour pressure deficit: The hidden driver behind plant morphofunctional traits in controlled environments. Ann. Appl. Biol. 2019, 175, 313–325. [Google Scholar] [CrossRef]
- Amitrano, C.; Arena, C.; Cirillo, V.; De Pascale, S.; De Micco, V. Leaf morpho-anatomical traits in Vigna radiata L. affect plant photosynthetic acclimation to changing vapor pressure deficit. Environ. Exp. Bot. 2021, 186, 104453. [Google Scholar] [CrossRef]
- Thiede, M.E.; Link, S.O.; Fellows, R.J.; Beedlow, P.A. Effect of gamma radiation on stem diameter growth, carbon gain and biomass partitioning in Helianthus annus. Environ. Exp. Bot. 1995, 35, 33–41. [Google Scholar] [CrossRef]
- Ursino, D.J.; Schefski, H.; McCabe, J. Radiation-induced changes in rate of photosynthetic CO2 uptake in soybean plants. Environ. Exp. Bot. 1977, 17, 27–34. [Google Scholar] [CrossRef]
- Jia, C.F.; Li, A.L. Effect of gamma radiation on mutant induction of Fagopyrum dibotrys Hara. Photosynthetica 2008, 46, 363–369. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Maheran, A.A.; Philip, E. Effects of Acute Gamma Irradiation on Physiological Traits and Flavonoid accumulation of Centella asiatica. Molecules 2011, 16, 4994–5007. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Shi, M.; Huang, J.Z.; Xu, J.; Wang, Z.D.; Guo, D.P. Regulation of photosynthetic performance and antioxidant capacity by 60Co γ-irradiation in Zizania latifolia plants. J. Environ. Radioact. 2014, 129, 33–42. [Google Scholar] [CrossRef]
- Akoyunoglou, G.; Anni, H. Blue light effect on chloroplast development in higher plants. In Blue Light Effects in Biological Systems; Senger, H., Ed.; Springer: Berlin, Germany, 1984; pp. 397–406. [Google Scholar]
- Sæbø, A.; Krekling, T.; Applegren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LED) with and without supplemental blue light. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, S.I.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hammeed, A.; Shah, T.M.; Atta, B.M.; Haq, M.A.; Sayed, H. Gamma Irradiation effects on seed germination and growth, protein content, peroxidase and protease activity, lipid peroxidation in Desi and Kabuli Chickpea. Pak. J. Bot. 2008, 40, 1033–1041. [Google Scholar]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological Responses of Orthosiphon stamineus Plantles to Gamma Irradiation. Am. Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- Zhang, L.; Liu, S.; Zhang, Z.; Yang, R.; Yang, X. Dynamic effects of different light qualities on pea sprouts quality. North. Hortic. 2010, 8, 4–7. [Google Scholar]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of Different Light Sources on the Growth of Non-heading Chinese Cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Amitrano, C.; Vitale, E.; De Micco, V.; Arena, C. Light fertilization affects growth and photosynthesis in mung bean (Vigna radiata) plants. J. Environ. Account. Manag. 2018, 6, 295–304. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.E.; Kodner, R.B.; Fisher, M.M.; Graham, J.M.; Wilcox, L.W.; Hackney, J.M.; Obst, J.; Bilkey, P.C.; Hanson, D.T.; Cook, M.E. Early land plant adaptations to terrestrial stress: A focus on phenolics. In The Evolution of Plant Physiology; Hemsley, A.R., Poole, I., Eds.; Elsevier: London, UK, 2004; pp. 165–168. [Google Scholar]
- Zhang, R.; Kang, K.A.; Kang, S.S.; Park, J.W. Morin (2′,3,4′,5,7-pentahydroxyflavone) protected cells against γ radiation-induced oxidative stress. Basic Clin. Pharmacol. Toxicol. 2011, 108, 63–72. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Mazzucato, A.; Olimpieri, I. Growth, yield and reproduction of dwarf tomato grown under simulated microgravity conditions. Plant Biosyst. 2007, 141, 75–81. [Google Scholar] [CrossRef]
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. TOMATOMA: A novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazama, Y.; Hirano, T.; Saito, H.; Liu, Y.; Ohbu, S.; Hayashi, Y.; Abe, T. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Feder, N.; O’brien, T. Plant microtechnique: Some principles and new methods. Am. J. Bot. 1968, 55, 123–142. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationship between the biochemistry of photosynthesis and the gas exchanges of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Yokota, K.; Kurashima, A.; Maegawa, M. High water temperature tolerance in photosynthetic activity of Zostera japonica Ascherson and Graebner seedlings from Ago Bay, Mio Prefecture, central Japan. Fish. Sci. 2009, 75, 1117–1123. [Google Scholar] [CrossRef]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A universal and rapid protocol for protein extraction from recalcitrant plant tissue for proteomic analysis. Electrophoresis 2006, 27, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersicum esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Guercia, G.; Turano, M.; Arena, C. Effects of different light quality and biofertilizers on structural and physiological traits of spinach plants. Photosynthetica 2020, 58, 932–943. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crop. Prod. 2017, 39, 74–80. [Google Scholar] [CrossRef]
- Sun, B.; da Silva, J.M.R.; Spranger, I. Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]







| Height | TLA | Leaves | Flowers | Fruits | |
|---|---|---|---|---|---|
| IR | |||||
| C | 7.1 a | 304 a | 10 b | 57 a | 24 a |
| IR | 5.5 b | 271 b | 11 a | 51 b | 23 a |
| LQ | |||||
| FL | 7.7 a | 323 a | 11 a | 50 b | 25 a |
| FS | 5.7 b | 276 b | 11 a | 50 b | 26 a |
| RB | 5.4 b | 263 b | 11 a | 62 a | 20 b |
| Significance | |||||
| IR | *** | *** | * | * | NS |
| LQ | *** | *** | NS | *** | ** |
| IR × LQ | * | * | *** | *** | NS |
| Functional Traits | Anatomical Traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LA | SLA | LDMC | RWC | LT | PT | ST | IS | SD | |
| IR | |||||||||
| C | 9.4 b | 210 a | 0.11 b | 60 b | 231 a | 99 a | 100 a | 31 a | 215 a |
| IR | 13 a | 219 a | 0.12 a | 66 a | 228 a | 94 a | 97 a | 33 a | 221 a |
| LQ | |||||||||
| FL | 13 a | 212 a | 0.12 a | 54 b | 188 c | 74 c | 81 c | 24 c | 213 a |
| FS | 10 b | 217 a | 0.11 a | 66 a | 221 b | 92 b | 94 b | 32 b | 222 a |
| RB | 9.8 b | 214 a | 0.11 a | 69 a | 280 a | 123 a | 120 a | 40 a | 217 a |
| Condition | |||||||||
| C-FL | 9.6 b | 219 a | 0.11 a | 55 d | 200 bc | 80 bc | 86 bc | 28 b | 181 a |
| IR-FL | 17 a | 205 a | 0.12 a | 53 d | 175 c | 68 c | 76 c | 21 c | 246 a |
| C-FS | 9.1 b | 203 a | 0.11 a | 62 c | 214 b | 92 b | 92 b | 30 b | 234 a |
| IR-FS | 12 b | 231 a | 0.11 a | 69 b | 228 b | 93 b | 96 b | 33 b | 210 a |
| C-RB | 9.5 b | 207 a | 0.11 a | 62 c | 280 a | 125 a | 120 a | 36 b | 229 a |
| IR-RB | 10 b | 222 a | 0.12 a | 75 a | 280 a | 121 a | 119 a | 48 a | 206 a |
| Significance | |||||||||
| IR | *** | NS | * | ** | NS | NS | NS | NS | NS |
| LQ | *** | NS | NS | NS | *** | *** | *** | *** | NS |
| IR × LQ | *** | NS | NS | * | NS | NS | NS | * | NS |
| PNsat | Isat | ΦCO2 | |
|---|---|---|---|
| IR | |||
| C | 23 a | 508 a | 0.047 a |
| IR | 25 a | 518 a | 0.050 a |
| LQ | |||
| FL | 23 b | 525 a | 0.044 b |
| FS | 26 a | 517 a | 0.051 a |
| RB | 25 a | 497 a | 0.052 a |
| Condition | |||
| C-FL | 25 b | 558 a | 0.045 b |
| IR-FL | 20 c | 492 a | 0.042 b |
| C-FS | 24 b | 509 a | 0.048 a |
| IR-FS | 27 a | 524 a | 0.053 a |
| C-RB | 22 c | 457 a | 0.049 a |
| IR-RB | 29 a | 537 a | 0.055 a |
| Significance | |||
| IR | NS | NS | NS |
| LQ | * | NS | ** |
| IR × LQ | *** | NS | * |
| TAC | TPC | FLAV | PROT | RUB | |
|---|---|---|---|---|---|
| IR | |||||
| C | 2.9 a | 0.92 a | 20 a | 0.30 b | 0.90 b |
| IR | 2.8 a | 0.83 b | 18 b | 0.41 a | 1.12 a |
| LQ | |||||
| FL | 2.6 b | 0.83 b | 18 b | 0.27 c | 0.92 b |
| FS | 3.0 a | 0.87 b | 19 b | 0.34 b | 0.81 b |
| RB | 2.9 a | 0.92 a | 20 a | 0.45 a | 1.30 a |
| Significance | |||||
| IR | NS | *** | *** | *** | * |
| LQ | *** | ** | ** | *** | ** |
| IR × LQ | *** | NS | NS | *** | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, E.; Vitale, L.; Costanzo, G.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation. Plants 2021, 10, 1752. https://doi.org/10.3390/plants10081752
Vitale E, Vitale L, Costanzo G, Velikova V, Tsonev T, Simoniello P, De Micco V, Arena C. Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation. Plants. 2021; 10(8):1752. https://doi.org/10.3390/plants10081752
Chicago/Turabian StyleVitale, Ermenegilda, Luca Vitale, Giulia Costanzo, Violeta Velikova, Tsonko Tsonev, Palma Simoniello, Veronica De Micco, and Carmen Arena. 2021. "Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation" Plants 10, no. 8: 1752. https://doi.org/10.3390/plants10081752
APA StyleVitale, E., Vitale, L., Costanzo, G., Velikova, V., Tsonev, T., Simoniello, P., De Micco, V., & Arena, C. (2021). Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation. Plants, 10(8), 1752. https://doi.org/10.3390/plants10081752