Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan
Abstract
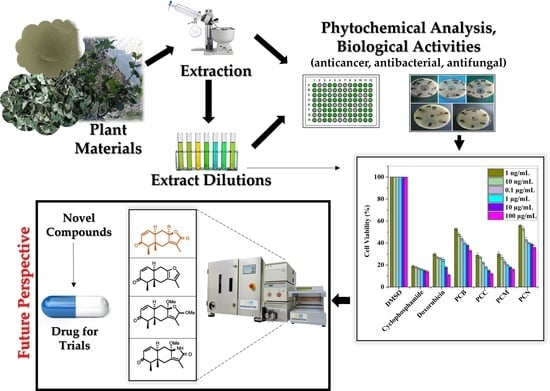
:1. Introduction
2. Results
2.1. Phytochemical Screening
2.2. Antimicrobial Potential
2.2.1. Antibacterial Effect
2.2.2. Antifungal Effect
2.3. Anticancer Activity






| Extracts (100 µg/mL) | Cancerous Cell Lines | Normal Cell Lines | |||||
|---|---|---|---|---|---|---|---|
| Caco-2 | A549 | HepG2 | MDA-MB-231 | NCI-H1437 | HPAEpiC | HRPTEpiC | |
| PCB | 42.6 | 26.85 | 50.3 | 19 | 67.71 | 64.85 | 58.85 |
| PCC | 20.5 | 25.42 | 37.3 | 18 | 58.14 | 72.28 | 52.28 |
| PCM | 22.3 | 29.14 | 34.6 | 26.57 | 47.71 | 78.85 | 63.28 |
| PCN | 44.5 | 22 | 30.5 | 19.71 | 39.14 | 80.71 | 53.28 |
| QSB | 29.71 | 38.8 | 35.71 | 29.28 | 46.42 | 71.14 | 56 |
| QSC | 30.42 | 36.6 | 33 | 22.42 | 35.85 | 75.71 | 54.85 |
| QSM | 34.42 | 38.8 | 31.85 | 28.57 | 48.85 | 76.14 | 61.85 |
| QSN | 24 | 30.8 | 23.85 | 20.28 | 68.71 | 64.14 | 54 |
| Doxo. | 20.8 | 20.50 | 10.85 | 19 | 27.42 | 49.57 | 19.57 |
| Cyclopho | 17.14 | 14.42 | 17.85 | 18 | 20.71 | 57.14 | 18.71 |
3. Discussion
4. Materials and Methods
4.1. Plant Samples Preparation
4.2. Phytochemical Screening
4.2.1. Test for Alkaloids
Mayer’s Reagent Test
Hager’s Test
4.2.2. Test for Saponins
4.2.3. Test for Flavonoids
4.2.4. Test for Tannins
Alkaline Reagent Test
Ferric Chloride Test
4.2.5. Test for Glycosides
4.2.6. Test for Sterols
4.2.7. Test for Phenols
Ellagic Test
4.2.8. Test for Carbohydrates
4.2.9. Test for Proteins
Xanthoproteic Test
4.2.10. Test for Anthraquinones
4.2.11. Test for Phlobatanins
4.3. Antimicrobial Assay
4.3.1. Antibacterial Activity
Bacterial Strains
Agar-Well Diffusion Method
4.3.2. Antifungal Activity
Fungal Strains
Agar-Well Diffusion Method
Agar Slanting Method
4.4. Anticancer and Safety Activities
4.4.1. Cancer Cell Lines and Primary Human Cell Culture
4.4.2. MTS Based Cell Viability Assay Procedure
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.–An overview. J. Ethnopharmacol. 2020, 253, 112667. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lalramnghinglova, H. Ethnomedicinal plant resources of Mizoram, India: Implication of traditional knowledge in health care system. Ethnobot. Leafl. 2010, 2010, 6. [Google Scholar]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M. Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Khanna, S. Immunological and biochemical markers in oral carcinogenesis: The public health perspective. Int. J. Environ. Res. Public Health 2008, 5, 418–422. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities, and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Iqbal, J. Impact of silvicultural system on natural regeneration in Western Himalayan moist temperate forests of Pakistan. J. For. Sci. 2021, 67, 101–112. [Google Scholar] [CrossRef]
- Sher, H.; Aldosari, A.; Ali, A.; de Boer, H.J. Indigenous knowledge of folk medicines among tribal minorities in Khyber Pakhtunkhwa, northwestern Pakistan. J. Ethnopharmacol. 2015, 166, 157–167. [Google Scholar] [CrossRef]
- Ahmad, H.; Öztürk, M.; Ahmad, W.; Khan, S.M. Status of Natural Resources in the Uplands of the Swat Valley Pakistan; Climate Change Impacts on High-Altitude Ecosystems; Springer: Berlin/Heidelberg, Germany, 2015; pp. 49–98. [Google Scholar]
- Jeelani, S.M.; Rather, G.A.; Sharma, A.; Lattoo, S.K. In perspective: Potential medicinal plant resources of Kashmir Himalayas, their domestication and cultivation for commercial exploitation. J. Appl. Res. Med. Aromat. Plants 2018, 8, 10–25. [Google Scholar] [CrossRef]
- Gilani, S.A.; Qureshi, R.A.; Khan, A.M.; Potter, D. Morphological characterization of the pollens of the selected species of genus Prunus Linn. from Northern Pakistan. Afr. J. Biotechnol. 2010, 9, 2872–2879. [Google Scholar]
- Söhretoglu, D.; Ekizoglu, M.; Kiliç, E.; Sakar, M.K. Antibacterial and antifungal activities of some Quercus species growing in Turkey. FABAD J. Pharm. Sci. 2007, 32, 127. [Google Scholar]
- Kausar, F.; Farooqi, M.-A.; Farooqi, H.-M.-U.; Salih, A.-R.-C.; Khalil, A.-A.-K.; Kang, C.-W.; Mahmoud, M.H.; Batiha, G.-E.-S.; Choi, K.-H.; Mumtaz, A.-S. Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled. Life 2021, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal uses, phytochemistry, and pharmacological activities of Quercus Species. Evid.-Based Complementary Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef]
- Balkrishan, A.; Tanwar, S.; Prajapati, U.B. Medicinal and Nutritional Aspect of Genus Prunus L. with Phytoetymology. Int. J. Unani Integr. Med. 2021, 5, 24–27. [Google Scholar]
- Alam, M.; Barua, R. In vitro regeneration and antibacterial activity of Prunus domestica L. J. BioSci. Biotechnol. 2015, 4, 9–15. [Google Scholar]
- Burlacu, E.; Nisca, A.; Tanase, C. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Sharba, Z.A.; Hasoon, B.A.; Maeah, R.K.; Hussein, N.N. Cytotoxicity, antioxidant, and antimicrobial activities of crude extract of quercus infectoria plant. Plant Arch. 2020, 20, 227–230. [Google Scholar]
- Jeong, M.; Kim, J.H.; Yang, H.; Dal Kang, S.; Song, S.; Lee, D.; Lee, J.S.; Park, J.H.Y.; Byun, S.; Lee, K.W. Heat-killed Lactobacillus plantarum KCTC 13314BP enhances phagocytic activity and immunomodulatory effects via activation of MAPK and STAT3 pathways. J. Microbiol. Biotechnol. 2019, 29, 1248–1254. [Google Scholar] [CrossRef]
- Abolhasani, M.H.; Safavi, M.; Goodarzi, M.T.; Kassaee, S.M.; Azin, M. Identification and anti-cancer activity in 2D and 3D cell culture evaluation of an Iranian isolated marine microalgae Picochlorum sp. RCC486. DARU J. Pharm. Sci. 2018, 26, 105–116. [Google Scholar] [CrossRef]
- Farooqi, H.M.U.; Khalid, M.A.U.; Kim, K.H.; Lee, S.R.; Choi, K.H. Real-time physiological sensor-based liver-on-chip device for monitoring drug toxicity. J. Micromech. Microeng. 2020, 30, 115013. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 1–19. [Google Scholar] [CrossRef]
- Murati, T.; Miletić, M.; Kolarić, J.; Lovrić, V.; Kovačević, D.B.; Putnik, P.; Landeka Jurčević, I.; Đikić, D.; Dragović-Uzelac, V.; Kmetič, I. Toxic activity of Prunus spinosa L. flower extract in hepatocarcinoma cells. Arhiv Higijenu Rada Toksikol. 2019, 70, 303–308. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Bandar, H.; Hijazi, A.; Rammal, H.; Hachem, A.; Saad, Z.; Badran, B. Techniques for the extraction of bioactive compounds from Lebanese Urtica Dioica. Am. J. Phytomed. Clin. Ther. 2013, 1, 507–513. [Google Scholar]
- Shah, M.D.; Hossain, M.A. Total flavonoids content and biochemical screening of the leaves of tropical endemic medicinal plant Merremia borneensis. Arab. J. Chem. 2014, 7, 1034–1038. [Google Scholar] [CrossRef]
- Yadav, M.; Chatterji, S.; Gupta, S.K.; Watal, G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci. 2014, 6, 539–542. [Google Scholar]
- Ali, S.; Khan, M.R.; Sajid, M.; Zahra, Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement. Altern. Med. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Archana, P.; Samatha, T.; Mahitha, B.; Chamundeswari, N.R. Preliminary phytochemical screening from leaf and seed extracts of Senna alata L. Roxb-an ethno medicinal plant. Int. J. Pharm. Biol. Res. 2012, 3, 82–89. [Google Scholar]
- Boyanova, L.; Gergova, G.; Nikolov, R.; Derejian, S.; Lazarova, E.; Katsarov, N.; Mitov, I.; Krastev, Z. Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J. Med. Microbiol. 2005, 54, 481–483. [Google Scholar] [CrossRef]
- Nasir, F.; Khan, M.S. Validation of Some of the Ethnopharmacological Uses of Xanthium strumarium and Duchesnea indica. Pak. J. Bot. 2012, 44, 1199–1201. [Google Scholar]
- Salih, A.R.C.; Farooqi, H.M.U.; Kim, Y.S.; Lee, S.H.; Choi, K.H. Impact of serum concentration in cell culture media on tight junction proteins within a multiorgan microphysiological system. Microelectron. Eng. 2020, 232, 111405. [Google Scholar] [CrossRef]
- Farooqi, H.M.U.; Kang, B.; Khalid, M.A.U.; Salih, A.R.C.; Hyun, K.; Park, S.H.; Huh, D.; Choi, K.H. Real-time monitoring of liver fibrosis through embedded sensors in a microphysiological system. Nano Converg. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Salih, A.R.C.; Hyun, K.; Asif, A.; Soomro, A.M.; Farooqi, H.M.U.; Kim, Y.S.; Kim, K.H.; Lee, J.W.; Huh, D.; Choi, K.H. Extracellular Matrix Optimization for Enhanced Physiological Relevance in Hepatic Tissue-Chips. Polymers 2021, 13, 3016. [Google Scholar] [CrossRef]
- Thakkar, K.; Prasad, A.; Nayak, J.; Iyer, S.V.; Kumar, S. Antioxidant and in vitro cytotoxic activity of extracts of aerial parts of Cocculus hirsutus (L) using cell line cultures (breast cell line). J. Phytopharm. 2014, 3, 395–399. [Google Scholar]
- Belhadj, F.; Somrani, I.; Aissaoui, N.; Messaoud, C.; Boussaid, M.; Marzouki, M.N. Bioactive compounds contents, antioxidant and antimicrobial activities during ripening of Prunus persica L. varieties from the North West of Tunisia. Food Chem. 2016, 204, 29–36. [Google Scholar] [CrossRef]

| Constituents | Tests | PCM | QSM |
|---|---|---|---|
| Alkaloids | Mayer’s test | + | + |
| Hager’s test | + | + | |
| Tannins | FCl3 test | + | + |
| Alkaline reagent test | + | + | |
| Saponins | Foam test | + | + |
| Flavonoids | + | + | |
| Glycosides | + | + | |
| Sterols | N | + | |
| Phenols | N | + | |
| Carbohydrates | N | N | |
| Anthraquinones | + | N | |
| Phlobatanins | − | − | |
| Anthocyanin | − | − | |
| Quinones | + | + | |
| Protein | Xanthoproteic test | N |
| Extract Solvents 4000 µg/mL | B. subtilis | E. coli | K. pneumoniae | S. enterica | A. baumannii |
|---|---|---|---|---|---|
| Zone of Inhibition (mm) | |||||
| PCB | 11.5 | 11.0 | 11.5 | 14.5 | 16 |
| PCC | 13 | 13 | 12 | 13 | 14 |
| PCE | 12 | 11.5 | 13 | 13 | 13 |
| PCM | 11 | 11 | 12 | 14 | 13 |
| PCN | 12 | 14 | 11 | 15.5 | 15 |
| QSB | 12 | 13 | 12 | 8 | 15 |
| QSC | 12.5 | 11 | 13 | 8 | 14 |
| QSE | 12 | 12.5 | 11 | 10 | 16 |
| QSM | 14 | 15 | 13 | 10 | 18 |
| QSN | 11 | 12.5 | 12.5 | 7 | 16 |
| P | 12 | 13 | 16 | 15 | 12 |
| N | - | - | - | - | - |
| Extract | R. oryzae | A. flavus | A. niger | Pythium sp. |
|---|---|---|---|---|
| Zone of Inhibition (mm) | ||||
| PCB | - | - | - | - |
| PCC | - | - | - | - |
| PCM | 16.5 | - | - | 1.5 |
| PCN | 16 | - | - | - |
| QSB | 16 | - | - | - |
| QSC | 16 | - | - | - |
| QSE | 21 | - | - | - |
| QSM | 16 | - | - | 2.25 |
| QSN | 16.5 | - | - | - |
| DMSO | - | - | - | - |
| Terbinafine | 30 | 35 | 32.5 | 36 |
| Extracts | Fungal Isolates | ||
|---|---|---|---|
| F. fujikuroi | R. oryzae | P. ultimum | |
| PCB | 54 | 62 | 38 |
| PCC | 59 | 59 | 39 |
| PCE | 52 | 64 | 40 |
| PCM | 55 | 67 | 43 |
| PCN | 50 | 60 | 44 |
| QSB | 49 | 57 | - |
| QSC | 54 | 53 | - |
| QSE | 46 | 54 | - |
| QSM | 44 | 57 | - |
| QSN | 37 | 48 | - |
| Positive control/Terbinafine | 56 | 79 | 62 |
| Negative control | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, F.; Kim, K.-H.; Farooqi, H.M.U.; Farooqi, M.A.; Kaleem, M.; Waqar, R.; Khalil, A.A.K.; Khuda, F.; Abdul Rahim, C.S.; Hyun, K.; et al. Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan. Plants 2022, 11, 48. https://doi.org/10.3390/plants11010048
Kausar F, Kim K-H, Farooqi HMU, Farooqi MA, Kaleem M, Waqar R, Khalil AAK, Khuda F, Abdul Rahim CS, Hyun K, et al. Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan. Plants. 2022; 11(1):48. https://doi.org/10.3390/plants11010048
Chicago/Turabian StyleKausar, Farzana, Kyung-Hwan Kim, Hafiz Muhammad Umer Farooqi, Muhammad Awais Farooqi, Muhammad Kaleem, Rooma Waqar, Atif Ali Khan Khalil, Fazli Khuda, Chethikkattuveli Salih Abdul Rahim, Kinam Hyun, and et al. 2022. "Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan" Plants 11, no. 1: 48. https://doi.org/10.3390/plants11010048
APA StyleKausar, F., Kim, K. -H., Farooqi, H. M. U., Farooqi, M. A., Kaleem, M., Waqar, R., Khalil, A. A. K., Khuda, F., Abdul Rahim, C. S., Hyun, K., Choi, K. -H., & Mumtaz, A. S. (2022). Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan. Plants, 11(1), 48. https://doi.org/10.3390/plants11010048