Effects of Plastic Film Mulching on Soil Enzyme Activities and Stoichiometry in Dryland Agroecosystems
Abstract
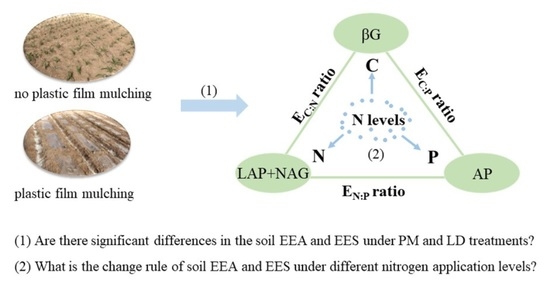
:1. Introduction
2. Results
2.1. Physical and Chemical Properties of Soil
2.2. Soil Extracellular Enzyme Activities and Its Stoichiometry
2.3. Relationship between Soil Physical and Chemical Properties and Soil Enzyme Activity and Its Stoichiometric Ratio
3. Discussion
3.1. Effects of Different Nitrogen Application Levels on Soil Physical and Chemical Properties under PM Treatments
3.2. Effects of Different Nitrogen Application Levels on Soil EEA under PM Treatments
3.3. Effects of Different Nitrogen Application Levels on Soil EES under Plastic Film Mulching Treatment
3.4. Soil Environmental Factors Related to Soil EEA and EES
4. Materials and Methods
4.1. Site Description
4.2. Experimental Design and Treatments
4.3. Soil Physiochemical Analysis
4.4. Soil Extracellular Enzyme Activity and Enzyme Stoichiometry Ratio
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.H.; Klironomos, J.N.; Henry, H.A.L. Seasonal Responses of Extracellular Enzyme Activity and Microbial Biomass to Warming and Nitrogen Addition. Soil Biol. Biochem. 2010, 74, 820–828. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, X. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Bio-Chem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a "Redfield ratio" for the microbial biomass? Biogeo-Chem. 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Han, F.; Ju, W.; Ye, L.; Wang, X.; Tan, W.; Zhang, X. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Hang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, W.; Martinez-Murillo, J.F.; Pereira, P. Loess Plateau: From degradation to restoration. Sci. Total Environ. 2020, 738, 140206. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, T.; Cai, T.; Ali, S.; Han, Q.F.; Ren, X.L.; Jia, Z.K. Plastic-Film Mulching for Enhanced Water-Use Efficiency and Economic Returns from Maize Fields in Semiarid China. Front. Plant Sci. 2017, 8, 512. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, J.; Xu, J.; Xu, H. Productivity and soil response to plastic film mulching durations for spring wheat on entisols in the semiarid Loess Plateau of China. Soil Tillage Res. 2004, 78, 9–20. [Google Scholar] [CrossRef]
- Roberts, T. Roles of fertilizer in growing world’s food. Better Crops Plant Food 2009, 93, 12–15. [Google Scholar]
- Peng, H.; Buresh, R.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q.; et al. Improving nitrogen fertilization in rice by site-specific N management. A review. Agron. Sustain. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Gao, S.; Wang, P.; Qiu, J.; Shang, S. Impacts of simulated nitrogen deposition on soil enzyme activity in a northern temperate forest ecosystem depend on the form and level of added nitrogen. Eur. J. Soil Biol. 2021, 103, 103287. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, J.; Zhou, X.; Cao, J.; Wang, R.; Wang, Y.; Shelt, S.; Jin, Z.; Walker, L.M.; et al. Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob. Change Biol. 2016, 23, 1328–1337. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Moorhead, D.L.; Sinsabaugh, R.L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Guo, T.; Xing, Y.; Mo, F.; Wang, H.; Fan, J.; Zhang, F. Effects of plastic mulch and nitrogen fertilizer on the soil microbial community, enzymatic activity and yield performance in a dryland maize cropping system. Eur. J. Soil Sci. 2020, 72, 149657. [Google Scholar] [CrossRef]
- Gao, H.; Yan, C.; Liu, Q.; Li, Z.; Yang, X.; Qi, R. Exploring optimal soil mulching to enhance yield and water use efficiency in maize cropping in China: A meta-analysis. Agric. Water Manag. 2019, 225, 105741. [Google Scholar] [CrossRef]
- Li, C.; Wen, X.; Wan, X.; Liu, Y.; Han, J.; Liao, Y.; Wu, W. Towards the highly effective use of precipitation by ridge-furrow with plastic film mulching instead of relying on irrigation resources in a dry semi-humid area. Field Crops Res. 2016, 188, 62–73. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.; Ma, B.; Xiong, Y.; Qiang, S.; Wang, C.; Liu, C.; Li, F. Ridge-furrow with full plastic film mulching improves water use efficiency and tuber yields of potato in a semiarid rainfed ecosystem. Field Crops Res. 2014, 161, 137–148. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, S.; Liu, C.; Xiong, Y.; Si, J.; Li, X.; Gan, Y.; Li, F. Ridge-furrow and plastic-mulching tillage enhances maize–soil interactions: Opportunities and challenges in a semiarid agroecosystem. Field Crops Res. 2012, 126, 181–188. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Wen, D.; Liu, X. Phosphatase activity in relation to key litter and soil properties in mature subtropical forests in China. Sci. Total Environ. 2015, 515, 83–91. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.P.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tian, H.; Wang, Z.; Liu, C.; Nurzhan, A.; Megharaj, M.; He, W. Potential effect of warming on soil microbial nutrient limitations as determined by enzymatic stoichiometry in the farmland from different climate zones. Sci. Total Environ. 2022, 802, 149657. [Google Scholar] [CrossRef]
- Lei, X.; Liu, G.; Li, P.; Xue, S. Ecological stoichiometry of plant-soil-enzyme interactions drives secondary plant succession in the abandoned grasslands of Loess Plateau, China. Gatena 2021, 202, 105302. [Google Scholar]
- Bao, S. Soil Agricultural Chemistry Analysis. China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Steinweg, J.; Dukes, J.; Wallenstein, M.D. Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol. Biochem. 2012, 55, 85–92. [Google Scholar] [CrossRef]






| pH | SOC | TN | TP | |
|---|---|---|---|---|
| S | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| P | =0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| N | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| S*P | =0.028 * | <0.001 ** | <0.001 ** | <0.001 ** |
| S*N | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| P*N | =0.023 * | =0.042 * | <0.001 ** | <0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhao, X.; Hossain, M.E.; Wang, S.; Dong, W.; Gopalakrishnan, S.; Liu, E. Effects of Plastic Film Mulching on Soil Enzyme Activities and Stoichiometry in Dryland Agroecosystems. Plants 2022, 11, 1748. https://doi.org/10.3390/plants11131748
Liu M, Zhao X, Hossain ME, Wang S, Dong W, Gopalakrishnan S, Liu E. Effects of Plastic Film Mulching on Soil Enzyme Activities and Stoichiometry in Dryland Agroecosystems. Plants. 2022; 11(13):1748. https://doi.org/10.3390/plants11131748
Chicago/Turabian StyleLiu, Meixia, Xueqing Zhao, Md Elias Hossain, Shangwen Wang, Wenyi Dong, Subramaniam Gopalakrishnan, and Enke Liu. 2022. "Effects of Plastic Film Mulching on Soil Enzyme Activities and Stoichiometry in Dryland Agroecosystems" Plants 11, no. 13: 1748. https://doi.org/10.3390/plants11131748
APA StyleLiu, M., Zhao, X., Hossain, M. E., Wang, S., Dong, W., Gopalakrishnan, S., & Liu, E. (2022). Effects of Plastic Film Mulching on Soil Enzyme Activities and Stoichiometry in Dryland Agroecosystems. Plants, 11(13), 1748. https://doi.org/10.3390/plants11131748