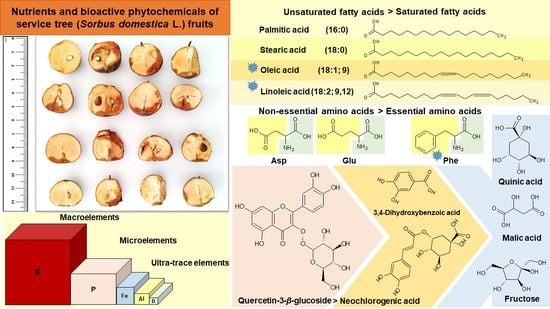
Nutrient Constituents, Bioactive Phytochemicals, and Antioxidant Properties of Service Tree (Sorbus domestica L.) Fruits
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein Content and Amino Acid Composition
2.2. Lipid Composition
2.3. Carbohydrate and Organic Acid Composition
2.4. Mineral Composition
2.5. Phenolic Compounds and Antioxidant Activities
3. Materials and Methods
3.1. Plant Material
3.2. Proximate Composition Analysis
3.3. Amino Acid Composition
3.4. Fatty Acid and Sterol Composition
3.5. Phospholipid and Tocopherol Content
3.6. Total Carotenoid and Chlorophyll Content
3.7. Uronic Acid, Cellulose, and Starch Content
3.8. High-Performance Liquid Chromatography Analysis of Available Carbohydrates
3.9. High-Performance Liquid Chromatography Analysis of Organic Acids
3.10. Mineral Composition
3.11. Total Phenolic, Flavonoid, and Condensed Tannin Content
3.12. High-Performance Liquid Chromatography of Phenolic Components
3.13. In Vitro Antioxidant Activity Assays
3.14. Lipid and Carbohydrate Indexes Calculation
3.15. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enescu, C.M.; de Rigo, D.; Durrant, T.H.; Caudillo, G. Sorbus domestica in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; European Commission: Luxembourg, 2016; p. e019db5+. [Google Scholar]
- Rotach, P. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Service Tree (Sorbus domestica); International Plant Genetic Resources Institute: Rome, Italy, 2003; 6p. [Google Scholar]
- George, J.P.; Konrad, H.; Collin, E.; Thevenet, J.; Ballian, D.; Idzojtic, M.; Kamm, U.; Zhelev, P.; Geburek, T. High molecular diversity in true service tree (Sorbus domestica) despite rareness: Data from Europe with special reference to the Austrian occurrence. Ann. Bot. 2015, 115, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brindza, J.; Červeňáková, J.; Tóth, D.; Bíro, D.; Sajbidor, J. Unutilized potential of true service tree (Sorbus domestica L.). ISHS Acta Hortic. 2009, 806, 717–726. [Google Scholar] [CrossRef]
- Mondeshka, P.K. Skorusha, 1st ed.; Zemizdat State Publishing House: Sofia, Bulgaria, 1990; pp. 5–67. [Google Scholar]
- Majić, B.; Šola, I.; Likić, S.; Cindrić, I.J.; Rusak, G. Characterisation of Sorbus domestica L. bark, fruits and seeds: Nutrient composition and antioxidant activity. Food Technol. Biotechnol. 2015, 53, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Mrkonjić, Z.; Nađpal, J.; Beara, I.; Šibul, F.; Knežević, P.; Lesjak, M.; Mimica-Dukić, N. Fresh fruits and jam of Sorbus domestica L. and Sorbus intermedia (Ehrh.) Pers.: Phenolic profiles, antioxidant action and antimicrobial activity. Bot. Serbica 2019, 43, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Piagnani, M.C.; Debellini, C.; LoScalzo, R. Phyllometry and carpometry, chemical and functional characterization of fruits of Sorbus domestica L. (service tree) selections. J. Berry Res. 2012, 2, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. Antioxidant activities of various extracts and fractions of Sorbus domestica fruits at different maturity stages. Food Chem. 2006, 98, 599–608. [Google Scholar] [CrossRef]
- Termentzi, A.; Alexiou, P.; Demopoulos, V.J.; Kokkalou, E. The aldose reductase inhibitory capacity of Sorbus domestica fruit extracts depends on their phenolic content and may be useful for the control of diabetic complications. Pharmazie 2008, 63, 693–696. [Google Scholar] [CrossRef]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. LC–DAD–MS (ESI+) analysis of the phenolic content of Sorbus domestica fruits in relation to their maturity stage. Food Chem. 2008, 106, 1234–1245. [Google Scholar] [CrossRef]
- Termentzi, A.; Zervou, M.; Kokkalou, E. Isolation and structure elucidation of novel phenolic constituents from Sorbus domestica fruits. Food Chem. 2009, 116, 371–381. [Google Scholar] [CrossRef]
- Zeiner, M.; Cindrić, I.J.; Majić, B.; Stingeder, G. Study of the accumulation of toxic and essential ultra-trace elements in fruits of Sorbus domestica L. Int. J. Environ. Res. Public Health 2017, 14, 341. [Google Scholar] [CrossRef] [Green Version]
- Akkol, E.K.; Dereli, F.T.G.; Taştan, H.; Sobarzo-Sánchez, E.; Khan, H. Effect of Sorbus domestica and its active constituents in an experimental model of colitis rats induced by acetic acid. J. Ethnopharmacol. 2020, 251, 112521. [Google Scholar] [CrossRef] [PubMed]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus domestica L. leaf extracts as functional products: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewska, M.A. Seasonal variation in phenylpropanoid biosynthesis and in vitro antioxidant activity of Sorbus domestica leaves: Harvesting time optimisation for medicinal application. Ind. Crops Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 8–428. [Google Scholar] [CrossRef]
- FAO Food and Nutrition Series—Collection. Amino-Acid Content of Foods and Biological Data on Proteins. 1981. Available online: http://www.fao.org/3/AC854T/AC854T00.htm#TOC (accessed on 24 May 2022).
- Alves, A.Q.; da Silva, V.A., Jr.; Góes, A.J.S.; Silva, M.S.; de Oliveira, G.G.; Bastos, I.V.G.A.; de Castro Neto, A.G.; Alves, A.J. The fatty acid composition of vegetable oils and their potential use in wound care. Adv. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive evaluation of late season peach varieties (Prunus persica L.): Fruit nutritional quality and phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Ognyanov, M.H.; Denev, P.N.; Kratchanova, M.G. Perspective therapeutic effects of immunomodulating acidic herbal heteropolysaccharides and their complexes in functional and dietary nutrition. In Therapeutic Foods, Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; Volume 8, pp. 285–327. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. In Horticultural Reviews; Warrington, I.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 45, pp. 371–430. [Google Scholar]
- Szentmihályi, K.; Kéry, Á.; Then, M.; Lakatos, L.; Sándor, Z.; Vinkler, P. Potassium–sodium ratio for the characterization of medicinal plant extracts with diuretic activity. Phytother. Res. 1998, 12, 163–166. [Google Scholar] [CrossRef]
- Harris, E.D. Minerals in Food: Nutrition, Metabolism, Bioactivity; DEStech Publications, Inc.: Lancaster, PA, USA, 2014; pp. 15–239. [Google Scholar]
- FAO; WHO. Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series, № 960; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Denev, P.; Lojek, P.; Ciz, M.; Kratchanova, M. Antioxidant activity and polyphenol content of Bulgarian fruits. Bulg. J. Agric. Sci. 2013, 19, 22–27. [Google Scholar]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- GB 5009.5-2016; Determination of Protein in Foods. National Food Safety Standard (NFSS) of the People’s Republic of China. China National Center for Food Safety Risk Assessment: Beijing, China, 2016.
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Petkova, Z.Y.; Antova, G.A.; Angelova-Romova, M.Y. Biologically active components and health benefits of nettle seed oil. Grasas Aceites 2020, 71, e347. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.B. Determination of starch, including maltooligosaccharides, in animal feeds: Comparison of methods and a method recommended for AOAC collaborative study. J. AOAC Int. 2009, 92, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolibdiphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Ivanov, I.G.; Vrancheva, R.Z.; Marchev, A.S.; Petkova, N.T.; Aneva, I.Y.; Denev, P.P.; Georgiev, V.G.; Pavlov, A.I. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 296–306. [Google Scholar]
| Crude Protein (N × 6.25), w/w% | 3.5 ± 0.2 | ||
|---|---|---|---|
| A. Essential amino acids | mg/g sample | mg/g N | g/100 g protein |
| Valine (Val) | 2.0 ± 0.3 | 357 | 5.8 |
| Leucine (Leu) | 1.9 ± 0.1 | 339 | 5.5 |
| Isoleucine (Ile) | 1.4 ± 0.2 | 250 | 3.9 |
| Phenylalanine (Phe) | 0.7 ± 0.08 | 125 | 2.1 |
| Tryptophan (Trp) | - | - | - |
| Methionine (Met) | 1.4 ± 0.1 | 250 | 4.1 |
| Threonine (Thr) | 1.1 ± 0.0 | 196 | 3.2 |
| Histidine * (His) | 1.6 ± 0.1 | 285 | 4.7 |
| Lysine (Lys) | 1.6 ± 0.1 | 285 | 4.6 |
| Arginine ** (Arg) | 2.1 ± 0.1 | 375 | 6.1 |
| Total | 13.8 | 2462 | 40 |
| B. Nonessential amino acids | |||
| Glycine (Gly) | 1.6 ± 0.2 | 285 | 4.6 |
| Alanine (Ala) | 2.4 ± 0.2 | 428 | 6.8 |
| Proline (Pro) | 2.4 ± 0.1 | 428 | 7.0 |
| Serine (Ser) | 1.7 ± 0.1 | 303 | 4.7 |
| Cysteine (Cys) | - | - | - |
| Tyrosine (Tyr) | 1.7 ± 0.2 | 303 | 4.9 |
| Asparagine (Asn) | - | - | - |
| Glutamine (Gln) | - | - | - |
| Aspartic acid (Asp) | 4.0 ± 0.2 | 714 | 11.2 |
| Glutamic acid (Glu) | 3.0 ± 0.1 | 535 | 8.7 |
| Total | 16.8 | 2996 | 47.9 |
| Total Lipids, w/w% | 0.82 ± 0.07 a | |
|---|---|---|
| A. Saturated fatty acids, % | 24.5 | |
| C8:0 | Caprylic acid | 0.4 ± 0.05 b |
| C10:0 | Capric acid | 0.2 ± 0.01 |
| C12:0 | Lauric acid | 0.1 ± 0.03 |
| C14:0 | Myristic acid | 0.4 ± 0.03 |
| C15:0 | Pentadecylic acid | 0.2 ± 0.03 |
| C16:0 | Palmitic acid | 19.5 ± 0.6 |
| C17:0 | Margaric acid | 0.3 ± 0.07 |
| C18:0 | Stearic acid | 3.3 ± 0.3 |
| C20:0 | Arachidic acid | 0.1 ± 0.02 |
| B. Unsaturated fatty acids, % | 75.5 | |
| C14:1 | Myristoleic acid | 0.1 ± 0.02 |
| C16:1 | Palmitoleic acid | 0.1 ± 0.04 |
| C18:1 | Oleic acid | 27.3 ± 0.3 |
| C18:2 | Linoleic acid | 47.0 ± 0.2 |
| C18:3 | Linolenic acid | 0.5 ± 0.2 |
| C20:3 | Eicosatrienoic acid (Dihomo-γ-linolenic acid) | 0.2 ± 0.02 |
| C20:4 | Eicosatetraenoic acid (Arachidonic acid) | 0.3 ± 0.04 |
| C. Sterols, % | 1.1 ± 0.1 | |
| Cholesterol | 1.4 ± 0.02 c | |
| Brassicasterol | 1.1 ± 0.04 | |
| Stigmasterol | 3.6 ± 0.1 | |
| β-Sitosterol | 90.2 ± 0.3 | |
| Δ5-Avenasterol | 0.6 ± 0.02 | |
| Δ7-Stigmasterol | 2.3 ± 0.1 | |
| Δ7-Avenasterol | 0.8 ± 0.1 | |
| D. Phospholipids, % | 3.6 ± 0.1 | |
| E. Tocopherols, mg/kg | 27.0 ± 2.0 | |
| F. Carotenoids, mg/kg | 7.6 ± 0.5 d | |
| G. Total chlorophylls, mg/kg | 37.8 ± 0.8 d | |
| Chlorophyll a | 13.7 ± 0.5 | |
| Chlorophyll b | 24.2 ± 0.3 | |
| A. Total Carbohydrate, w/w% | 44.4 ± 1.7 |
|---|---|
| Cellulose | 2.3 ± 0.2 |
| Starch | 1.1 ± 0.1 |
| Uronic acids (anhydrous) | 3.1 ± 0.3 |
| Glucose (Glc) | 13.0 ± 1.3 |
| Fructose (Fru) | 18.2 ± 1.1 |
| Sucrose (Suc) | 1.5 ± 0.1 |
| Sorbitol | 1.6 ± 0.1 |
| B. Organic acids, mg/100 g | |
| Malic acid | 1160 ± 130 |
| Quinic acid | 363 ± 39 |
| Ascorbic acid | <50 a |
| Shikimic acid | <25 a |
| Citric acid | <50 a |
| α-Ketoglutaric acid | <50 a |
| Tartaric acid | 44 ± 5 |
| C. Total titratable acidity (g malic acid/100 g) | 1.5 |
| Total Ash, w/w% | 0.98 ± 0.01 |
|---|---|
| Macroelements | Content, mg/kg |
| Potassium (K) | 4986.5 ± 85.5 |
| Phosphorus (P) | 400.0 ± 15.5 |
| Magnesium (Mg) | 40.9 ± 2.1 |
| Calcium (Ca) | 31.6 ± 1.8 |
| Sodium (Na) | 10.0 ± 1.0 |
| Micro (Trace) Elements | |
| Iron (Fe) | 40.6 ± 2.5 |
| Copper (Cu) | 2.2 ± 0.2 |
| Zinc (Zn) | 1.3 ± 0.2 |
| Manganese (Mn) | 0.9 ± 0.1 |
| Chromium (Cr) | 0.12 ± 0.02 |
| Selenium (Se) | <0.1 |
| Cobalt (Co) | 0.07 ± 0.01 |
| Molybdenum (Mo) | 0.01 ± 0.00 |
| Nickel (Ni) | 0.06 ± 0.001 a |
| Ultra-Trace and Toxic Elements | |
| Aluminium (Al) | 13.0 ± 2.0 |
| Boron (B) | 5.4 ± 0.4 |
| Barium (Ba) | 4.0 ± 0.6 |
| Strontium (Sr) | 3.2 ± 0.4 |
| Arsenic (As) | <0.05 |
| Cadmium (Cd) | 0.05 ± 0.005 |
| Mercury (Hg) | <0.03 |
| Lead (Pb) | 0.02 ± 0.001 |
| Bismuth (Bi) | - |
| A. Total Phenolic Content, mg GAE/100 g | 3574 ± 20 |
|---|---|
| B. Total flavonoids content, mg QE/100 g | 76.1 ± 1.0 |
| Quercetin, mg/100 g | 6.1 ± 0 |
| Quercetin-3-β-glucoside | 146 ± 12 |
| Epicatechin | 74 ± 2 |
| Rutin | 65 ± 2 |
| Naringin | 53 ± 7 |
| Myricetin | 40 ± 1 |
| C. Total tannins, mg/100 g | 122 ± 10 |
| D. Phenolic acids, mg/100 g | |
| Neochlorogenic acid | 908 ± 35 |
| 3,4-Dihydroxybenzoic acid | 886 ± 15 |
| Ellagic acid | 26 ± 1 |
| Ferulic acid | 22 ± 1 |
| E. Antioxidant activity | |
| DPPH assay, mM TE/g | 11.3 ± 1.9 |
| FRAP assay, mM TE/g | 9.4 ± 0.3 |
| ORAC assay, µmol TE/g | 642.3 ± 31.9 |
| HORAC assay, µmol GAE/g | 67.8 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ognyanov, M.; Denev, P.; Petkova, N.; Petkova, Z.; Stoyanova, M.; Zhelev, P.; Matev, G.; Teneva, D.; Georgiev, Y. Nutrient Constituents, Bioactive Phytochemicals, and Antioxidant Properties of Service Tree (Sorbus domestica L.) Fruits. Plants 2022, 11, 1832. https://doi.org/10.3390/plants11141832
Ognyanov M, Denev P, Petkova N, Petkova Z, Stoyanova M, Zhelev P, Matev G, Teneva D, Georgiev Y. Nutrient Constituents, Bioactive Phytochemicals, and Antioxidant Properties of Service Tree (Sorbus domestica L.) Fruits. Plants. 2022; 11(14):1832. https://doi.org/10.3390/plants11141832
Chicago/Turabian StyleOgnyanov, Manol, Petko Denev, Nadezhda Petkova, Zhana Petkova, Magdalena Stoyanova, Peter Zhelev, Georgi Matev, Desislava Teneva, and Yordan Georgiev. 2022. "Nutrient Constituents, Bioactive Phytochemicals, and Antioxidant Properties of Service Tree (Sorbus domestica L.) Fruits" Plants 11, no. 14: 1832. https://doi.org/10.3390/plants11141832
APA StyleOgnyanov, M., Denev, P., Petkova, N., Petkova, Z., Stoyanova, M., Zhelev, P., Matev, G., Teneva, D., & Georgiev, Y. (2022). Nutrient Constituents, Bioactive Phytochemicals, and Antioxidant Properties of Service Tree (Sorbus domestica L.) Fruits. Plants, 11(14), 1832. https://doi.org/10.3390/plants11141832