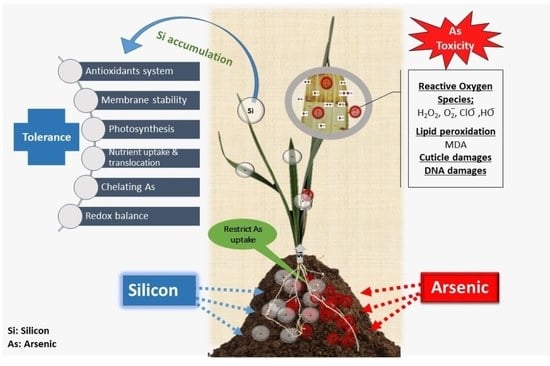
Silicon-Induced Tolerance against Arsenic Toxicity by Activating Physiological, Anatomical and Biochemical Regulation in Phoenix dactylifera (Date Palm)
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Growth and Treatment with Silicon and Arsenic
2.2. Quantification of Photosynthetic Pigments
2.3. Determination of Leaf Water Content
2.4. Evaluation of Protein Content and Antioxidant Enzymes
2.5. Determination of MDA Levels
2.6. Determination of Superoxide Anion (O2•−)
2.7. Elemental Analysis by Inductively-Coupled Plasma–Mass Spectrometry (ICP–MS)
2.8. Microscopic Analysis of Date Palm Shoot and Root Samples
2.9. Phytohormone Extraction and Quantification
2.10. Organic Acids Extraction and Quantification
2.11. RNA Extraction and cDNA Synthesis
2.12. qRT–PCR Gene Expression Profiling
2.13. Statistical Analysis
3. Results
3.1. Effects of Silicon and Arsenic on Growth Attributes
3.2. Determination of Chlorophyll Contents and RWC
3.3. Influence of Silicon on the Antioxidant System of Date Palm under Arsenic Stress
3.4. Estimation of MDA and Superoxide Anion Levels
3.5. Influence of Silicon on Phytohormones
3.6. Modulation of Organic Acids Levels by Si
3.7. Influence of Silicon on Plant Anatomy in Stress
3.8. Determination of Endogenous Si and Arsenic Levels
3.9. Expression of Abiotic Stress-Associated Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Muchuweti, M.; Birkett, J.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Kashif, M.; Sattar, A.; Ul-Allah, S.; Sher, A.; Ijaz, M.; Butt, M.; Qayyum, A. Silicon Alleviates Arsenic Toxicity in Maize Seedlings by Regulating Physiological and Antioxidant Defense Mechanisms. J. Soil Sci. Plant Nutr. 2021, 21, 2032–2040. [Google Scholar] [CrossRef]
- Salama, K.F.; Randhawa, M.A.; Al Mulla, A.A.; Labib, O.A. Heavy metals in some date palm fruit cultivars in Saudi Arabia and their health risk assessment. Int. J. Food Prop. 2019, 22, 1684–1692. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Xu, W.; Dai, W.; Yan, H.; Li, S.; Shen, H.; Chen, Y.; Xu, H.; Sun, Y.; He, Z.; Ma, M. Arabidopsis NIP3; 1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol. Plant 2015, 8, 722–733. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sengupta, M.K.; Ahamed, S.; Chowdhury, U.K.; Hossain, M.A.; Das, B.; Lodh, D.; Saha, K.C.; Pati, S.; Kaies, I. The magnitude of arsenic contamination in groundwater and its health effects to the inhabitants of the Jalangi—one of the 85 arsenic affected blocks in West Bengal, India. Sci. Total Environ. 2005, 338, 189–200. [Google Scholar] [CrossRef]
- Nickson, R.; McArthur, J.; Burgess, W.; Ahmed, K.M.; Ravenscroft, P.; Rahmann, M. Arsenic poisoning of Bangladesh groundwater. Nature 1998, 395, 338. [Google Scholar] [CrossRef] [PubMed]
- Sun, G. Arsenic contamination and arsenicosis in China. Toxicol. Appl. Pharmacol. 2004, 198, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Nasir, M.; Zhang, Y.; Gao, J.; Lv, Y.; Lv, J. Comparison of DGT with traditional extraction methods for assessing arsenic bioavailability to Brassica chinensis in different soils. Chemosphere 2018, 191, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Suriyagoda, L.D.; Dittert, K.; Lambers, H. Arsenic in Rice soils and potential agronomic mitigation strategies to reduce arsenic bioavailability: A review. Pedosphere 2018, 28, 363–382. [Google Scholar] [CrossRef]
- Li, Y.; Ye, F.; Wang, A.; Wang, D.; Yang, B.; Zheng, Q.; Sun, G.; Gao, X. Chronic arsenic poisoning probably caused by arsenic-based pesticides: Findings from an investigation study of a household. Int. J. Environ. Res. Public Health 2016, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mapa, R.B. Environmental soil issues. In The Soils of Sri Lanka; Springer: New York, NY, USA, 2020; pp. 119–124. [Google Scholar]
- Abedi, T.; Mojiri, A. Arsenic uptake and accumulation mechanisms in rice species. Plants 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Castor, J.; Guzmán-Mar, J.; Hernández-Ramírez, A.; Garza-González, M.; Hinojosa-Reyes, L. Arsenic accumulation in maize crop (Zea mays): A review. Sci. Total Environ. 2014, 488, 176–187. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- Sharma, S. Mechanisms of silicon for abiotic stress tolerance in higher plants: A review. Pharma Innov. J. 2022, 11, 1647–1657. [Google Scholar]
- Khaliq, A.; Ali, S.; Hameed, A.; Farooq, M.A.; Farid, M.; Shakoor, M.B.; Mahmood, K.; Ishaque, W.; Rizwan, M. Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis, and suppressing Ni uptake and oxidative stress. Arch. Agron. Soil Sci. 2016, 62, 633–647. [Google Scholar] [CrossRef]
- Ashfaque, F.; Inam, A.; Iqbal, S.; Sahay, S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S. Afr. J. Bot. 2017, 111, 153–160. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, S.; Han, D.; Zhou, Z.; Li, X.; Liu, Y.; Zhang, B. Silicon alleviates cadmium toxicity in wheat seedlings (Triticum aestivum L.) by reducing cadmium ion uptake and enhancing antioxidative capacity. Environ. Sci. Pollut. Res. 2018, 25, 7638–7646. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Shivaraj, S.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Gheshlaghpour, J.; Asghari, B.; Khademian, R.; Sedaghati, B. Silicon alleviates cadmium stress in basil (Ocimum basilicum L.) through alteration of phytochemical and physiological characteristics. Ind. Crops Prod. 2021, 163, 113338. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon mechanisms to ameliorate heavy metal stress in plants. BioMed Res. Int. 2018, 2018, 8492898. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.-D.; Miche, H.; Keller, C. Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J. Hazard. Mater. 2012, 209, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, C.; Fu, Y.; Wu, D.; Yang, X.; Shen, H. Silicate-mediated alleviation of Pb toxicity in banana grown in Pb-contaminated soil. Biol. Trace Elem. Res. 2012, 145, 101–108. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, L.R.; Batista, B.L.; da Silva Lobato, A.K. Silicon reduces aluminum accumulation and mitigates toxic effects in cowpea plants. Acta Physiol. Plant. 2017, 39, 138. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Kumar, D.; Chauhan, D.K. Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol. Plant. 2012, 34, 279–289. [Google Scholar] [CrossRef]
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M. Therapeutic potential of date palm against human infertility: A review. Metabolites 2021, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Kumar, P.P. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 2015, 6, 348. [Google Scholar] [CrossRef] [PubMed]
- El-Juhany, L.I. Degradation of date palm trees and date production in Arab countries: Causes and potential rehabilitation. Aust. J. Basic Appl. Sci. 2010, 4, 3998–4010. [Google Scholar]
- Abdulaal, W.H.; Zeyadi, M.; Baothman, O.A.; Zamzami, M.A.; Choudhry, H.; Almulaiky, Y.Q.; Saleh, R.M.; Mohamed, S.A. Investigation of antioxidant and detoxifying capacities of some date cultivars (Phoenix dactylifera L.) irrigated with sewage water. RSC Adv. 2017, 7, 12953–12958. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Shahroona, B.; Al-Yahyai, R.; Ahmed, M. Heavy metal concentrations in soils and date palms irrigated by groundwater and treated wastewater. Pak. J. Agric. Sci. 2015, 52, 129–134. [Google Scholar]
- Chaâbene, Z.; Rorat, A.; Hakim, I.R.; Bernard, F.; Douglas, G.C.; Elleuch, A.; Vandenbulcke, F.; Mejdoub, H. Insight into the expression variation of metal-responsive genes in the seedling of date palm (Phoenix dactylifera). Chemosphere 2018, 197, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.A. Growth and heavy metals uptake by date palm grown in mono-and dual culture in heavy metals contaminated soil. World Appl. Sci. J. 2011, 15, 429–435. [Google Scholar]
- Alansi, S.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Tarroum, M.; Alshameri, A.; Gaafar, A.-R.Z. Cryopreservation: A tool to conserve date palm in Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Mesnoua, M.; Roumani, M.; Mizab, O.; Zeguerrou, R. Heavy metals differentially affect date palm pollen germination and tube elongation. Italus Hortus 2020, 27, 64–71. [Google Scholar] [CrossRef]
- Awad, K.M.; Salih, A.M.; Khalaf, Y.; Suhim, A.A.; Abass, M.H. Phytotoxic and genotoxic effect of Aluminum to date palm (Phoenix dactylifera L.) in vitro cultures. J. Genet. Eng. Biotechnol. 2019, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Khan, A.; Imran, M.; Khan, A.L.; Asaf, S.; Al-Rawahi, A.; Al-Azri, M.S.A.; Al-Harrasi, A.; Lee, I.-J. Silicon-and Boron-Induced Physio-Biochemical Alteration and Organic Acid Regulation Mitigates Aluminum Phytotoxicity in Date Palm Seedlings. Antioxidants 2022, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606X. [Google Scholar]
- Cao, Y.-Y.; Yang, M.-T.; Chen, S.-Y.; Zhou, Z.-Q.; Li, X.; Wang, X.-J.; Bai, J.-G. Exogenous sucrose influences antioxidant enzyme activities and reduces lipid peroxidation in water-stressed cucumber leaves. Biol. Plant. 2015, 59, 147–153. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Lee, I.-J. Synergistic interaction of fungal endophytes, Paecilomyces formosus LHL10 and Penicillium funiculosum LHL06, in alleviating multi-metal toxicity stress in Glycine max L. Environ. Sci. Pollut. Res. 2021, 28, 67429–67444. [Google Scholar] [CrossRef] [PubMed]
- Okaichi, Y.; Ishikura, Y.; Akimoto, K.; Kawashima, H.; Toyoda-Ono, Y.; Kiso, Y.; Okaichi, H. Arachidonic acid improves aged rats’ spatial cognition. Physiol. Behav. 2005, 84, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Rehman, N.U.; Khan, A.L.; Al-Broumi, M.; Al-Amri, I.; Hussain, J.; Hussain, H.; Csuk, R. Chemical, molecular and structural studies of Boswellia species: β-Boswellic Aldehyde and 3-epi-11β-Dihydroxy BA as precursors in biosynthesis of boswellic acids. PLoS ONE 2018, 13, e0198666. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Waqas, M.; Ullah, I.; Bilal, S.; Kim, Y.-H.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Metabolic and proteomic alteration in phytohormone-producing endophytic Bacillus amyloliquefaciens RWL-1 during methanol utilization. Metabolomics 2019, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Endophytic Paecilomyces formosus LHL10 augments Glycine max L. adaptation to Ni-contamination through affecting endogenous phytohormones and oxidative stress. Front. Plant Sci. 2017, 8, 870. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, R.; Yu, N.; Zhang, W.; Xing, L.; Xie, D.; Peng, D. A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS ONE 2018, 13, e0196592. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Andreazza, R.; Da Ros, C.; Dellai, A.; Jacques, R.; Scheid, D. Growth of tropical tree species and absorption of copper in soil artificially contaminated. Braz. J. Biol. 2015, 75, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Panaullah, G.M.; Alam, T.; Hossain, M.B.; Loeppert, R.H.; Lauren, J.G.; Meisner, C.A.; Ahmed, Z.U.; Duxbury, J.M. Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 2009, 317, 31–39. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Fendorf, S. Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 13176–13183. [Google Scholar] [CrossRef]
- Muneer, S.; Park, Y.G.; Kim, S.; Jeong, B.R. Foliar or subirrigation silicon supply mitigates high temperature stress in strawberry by maintaining photosynthetic and stress-responsive proteins. J. Plant Growth Regul. 2017, 36, 836–845. [Google Scholar] [CrossRef]
- Nugues, M.; Roberts, C. Coral mortality and interaction with algae in relation to sedimentation. Coral Reefs 2003, 22, 507–516. [Google Scholar] [CrossRef]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef]
- Duman, F.; Ozturk, F.; Aydin, Z. Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As (III) and As (V): Effects of concentration and duration of exposure. Ecotoxicology 2010, 19, 983–993. [Google Scholar] [CrossRef]
- Stoeva, N.; Bineva, T. Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulg. J. Plant Physiol. 2003, 29, 87–95. [Google Scholar]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, R.D.; Singh, R.P.; Dwivedi, S.; Goutam, D.; Shri, M.; Trivedi, P.K.; Chakrabarty, D. Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol. Eng. 2013, 52, 96–103. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, P.; Sharma, Y.K. Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J. Plant Nutr. 2017, 40, 298–306. [Google Scholar] [CrossRef]
- Maglovski, M.; Gersi, Z.; Rybansky, L.; Bardacova, M.; Moravcikova, J.; Bujdos, M.; Dobrikova, A.; Apostolova, E.; Kraic, J.; Blehová, A. Effects of nutrition on wheat photosynthetic pigment responses to arsenic stress. Pol. J. Environ. Stud. 2019, 28, 1821–1829. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Shim, J.K.; Kim, D.H.; Lee, K.Y.; Lee, I.J. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J. Plant Growth Regul. 2014, 33, 137–149. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K.; Maathuis, F.J. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, M.; Lyubun, Y.V.; Kosterin, P.V.; Dudel, E.G. Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ. Toxicol. Int. J. 2004, 19, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Telles Nascimento, K.J.; Debona, D.; Silveira, P.R.; Silva, L.C.; DaMatta, F.M.; Rodrigues, F.Á. Silicon-induced changes in the antioxidant system reduce soybean resistance to frogeye leaf spot. J. Phytopathol. 2016, 164, 768–778. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Shahzad, R.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Azhri, M.; Mohanta, T.K.; Lee, I.-J. Silicon and gibberellins: Synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 2020, 9, 620. [Google Scholar] [CrossRef]
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2019, 129, 525–535. [Google Scholar] [CrossRef]
- Bari, M.; Prity, S.; Das, U.; Akther, M.; Sajib, S.; Reza, M.; Kabir, A. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020, 22, 472–479. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, L.; Wang, Q.; Fan, Z.; Kong, J.; Bai, X. Effects of exogenous nitric oxide on photosynthesis, antioxidative ability, and mineral element contents of perennial ryegrass under copper stress. J. Plant Interact. 2014, 9, 402–411. [Google Scholar] [CrossRef]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Qin, X.; Zeevaart, J.A. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Mou, K.; Dan, Y.; Li, W.; Wang, C.; Liao, W. The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Sci. Hortic. 2022, 292, 110631. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Malik, R.N.; Frantz, A.; Jaspers, V.L.B. A review on current knowledge and future prospects of organohalogen contaminants (OHCs) in Asian birds. Sci. Total Environ. 2016, 542, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Meguro, A.; Sato, Y. Salicylic acid antagonizes abscisic acid inhibition of shoot growth and cell cycle progression in rice. Sci. Rep. 2014, 4, 4555. [Google Scholar] [CrossRef]
- Gao, S.; Yan, R.; Cao, M.; Yang, W.; Wang, S.; Chen, F. Effects of copper on growth, antioxidant enzymes and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedling. Plant Soil Environ. 2008, 54, 117. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativalow silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Havshøi, N.W.; Fuglsang, A.T. A critical review on natural compounds interacting with the plant plasma membrane H+-ATPase and their potential as biologicals in agriculture. J. Integr. Plant Biol. 2022, 64, 268–286. [Google Scholar] [CrossRef]
- Chen, W.; Jia, P.F.; Yang, W.C.; Li, H.J. Plasma membrane H+-ATPases-mediated cytosolic proton gradient regulates pollen tube growth. J. Integr. Plant Biol. 2020, 62, 1817–1822. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, M.; Zeng, H.; Hayashi, Y.; Zhu, Y.; Kinoshita, T. Molecular basis of plasma membrane H+-ATPase function and potential application in the agricultural production. Plant Physiol. Biochem. 2021, 168, 10–16. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Yang, Y. The molecular mechanism of plasma membrane H+-ATPases in plant responses to abiotic stress. J. Genet. Genom. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nobori, T. Closing the gap: A plasma membrane H+-ATPase regulates stomatal closure. Plant Cell 2022, 34, 2582–2583. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, S.; Li, Y.; Zheng, F.; He, B.; Gu, M. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. 2020, 27, 8719–8731. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F. Arsenic tolerance in plants:“Pas de deux” between phytochelatin synthesis and ABCC vacuolar transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 20853–20854. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Geisler, M.J.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef]
- Mishra, S.; Mattusch, J.; Wennrich, R. Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol-complexation to restrict their translocation to shoot. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Yu, S.; Sun, Q.; Wu, J.; Zhao, P.; Sun, Y.; Guo, Z. Genome-Wide Identification and Characterization of Short-Chain Dehydrogenase/Reductase (SDR) Gene Family in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9498. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, E.; Hawkins, S.; Van Doorsselaere, J.; Piquemal, J.; Goffner, D.; Poeydomenge, O.; Boudet, A.M.; Grima-Pettenati, J. Cinnamoyl CoA reductase, the first committed enzyme of the lignin branch biosynthetic pathway: Cloning, expression and phylogenetic relationships. Plant J. 1997, 11, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Paddock, T.N.; Mason, M.E.; Lima, D.F.; Armstrong, G.A. Arabidopsis protochlorophyllide oxidoreductase A (PORA) restores bulk chlorophyll synthesis and normal development to a porB porC double mutant. Plant Mol. Biol. 2010, 72, 445–457. [Google Scholar] [CrossRef]
- Rahier, A.; Bergdoll, M.; Génot, G.; Bouvier, F.; Camara, B. Homology Modeling and Site-Directed Mutagenesis Reveal Catalytic Key Amino Acids of 3 β-Hydroxysteroid-Dehydrogenase/C4-Decarboxylase from Arabidopsis. Plant Physiol. 2009, 149, 1872–1886. [Google Scholar] [CrossRef]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-G.; Lin, N.-C.; Hsiao, Y.-Y.; Kuo, C.-H.; Chang, P.-F.; Deng, W.-L.; Chiang, M.-H.; Shen, H.-L.; Chen, C.-Y.; Cheng, W.-H. The Arabidopsis short-chain dehydrogenase/reductase 3, an abscisic acid deficient 2 homolog, is involved in plant defense responses but not in ABA biosynthesis. Plant Physiol. Biochem. 2012, 51, 63–73. [Google Scholar] [CrossRef] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.; Bilal, S.; Asaf, S.; Alamri, S.S.; Imran, M.; Khan, A.L.; Al-Rawahi, A.; Lee, I.-J.; Al-Harrasi, A. Silicon-Induced Tolerance against Arsenic Toxicity by Activating Physiological, Anatomical and Biochemical Regulation in Phoenix dactylifera (Date Palm). Plants 2022, 11, 2263. https://doi.org/10.3390/plants11172263
Khan T, Bilal S, Asaf S, Alamri SS, Imran M, Khan AL, Al-Rawahi A, Lee I-J, Al-Harrasi A. Silicon-Induced Tolerance against Arsenic Toxicity by Activating Physiological, Anatomical and Biochemical Regulation in Phoenix dactylifera (Date Palm). Plants. 2022; 11(17):2263. https://doi.org/10.3390/plants11172263
Chicago/Turabian StyleKhan, Taimoor, Saqib Bilal, Sajjad Asaf, Safiya Salim Alamri, Muhammad Imran, Abdul Latif Khan, Ahmed Al-Rawahi, In-Jung Lee, and Ahmed Al-Harrasi. 2022. "Silicon-Induced Tolerance against Arsenic Toxicity by Activating Physiological, Anatomical and Biochemical Regulation in Phoenix dactylifera (Date Palm)" Plants 11, no. 17: 2263. https://doi.org/10.3390/plants11172263
APA StyleKhan, T., Bilal, S., Asaf, S., Alamri, S. S., Imran, M., Khan, A. L., Al-Rawahi, A., Lee, I. -J., & Al-Harrasi, A. (2022). Silicon-Induced Tolerance against Arsenic Toxicity by Activating Physiological, Anatomical and Biochemical Regulation in Phoenix dactylifera (Date Palm). Plants, 11(17), 2263. https://doi.org/10.3390/plants11172263