Anti-Apoptotic and Anti-Inflammatory Effects of an Ethanolic Extract of Lycium chinense Root against Particulate Matter 10-Induced Cell Death and Inflammation in RBL-2H3 Basophil Cells and BALB/c Mice
Abstract
:1. Introduction
2. Results
2.1. Effects of PM10 and GR30 on RBL-2H3 Cell Viability
2.2. Effects of PM10 and GR30 on Cell Cycle Arrest and DNA Fragmentation
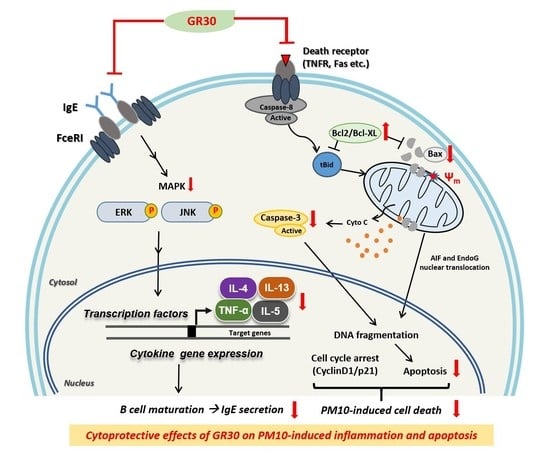
2.3. GR30 Antagonizes PM10-Induced Apoptotic Cell Death via Mitochondria-Dependent Apoptosis in RBL-2H3 Cells
2.4. GR30 Suppresses PM10-Induced Inflammatory Cytokine and COX2 Secretion in Basophils
2.5. GR30 Downregulates the PM10-Induced MAPK Pathway Activation
2.6. GR30 Suppresses PM10-Induced Serum IgE Production and TNF-α and Bax Expression in Lung Tissues
2.7. Chemical Profile of GR30 Determined by HPLC and LC-MS Analysis
3. Discussion
4. Methods and Materials
4.1. Materials
4.2. Preparation and Major Chemical Profile of GR30
4.3. Cell Culture and Cytotoxicity Assay
4.4. Animals, Diets and Experimental Protocol
4.5. Total RNA Extraction and Reverse Transcription PCR (RT-PCR)
4.6. Western Blot Analysis
4.7. TUNEL Assay
4.8. Measurement of IgE Production
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERK | extracellular signal-regulated kinase |
| GR30 | ethanol extract of Lycium chinense Miller root |
| HPLC | high-performance liquid chromatography |
| IgE | immunoglobulin E |
| IL-4 | interleukin 4 |
| IL-13 | interleukin 13 |
| JNK | the stress-activated c-jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MEM | minimum essential medium |
| LC-MS | liquid chromatography-mass spectrometry |
| PI | propidium iodide |
| PM10 | particulate matter 10 |
| TNF-α | tumour necrosis factor-α |
| TUNEL | terminal deoxynucleotidyltransferase (TdT) dUTP nick end labelling |
| UHPLC | ultra-high-performance liquid chromatography |
| COX2 | cyclooxygenase 2 |
References
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.M.; Lindsay, M.A. Apoptosis as a therapeutic target for the treatment of lung disease. Curr. Opin. Pharmacol. 2005, 5, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, K.E.; Carter, J.M.; Hassenbein, D.G.; Howard, B. Cytokines and particle-induced inflammatory cell recruitment. Environ. Health Perspect 1997, 105 (Suppl. 5), 1159–1164. [Google Scholar]
- Kodavanti, U.P.; Schladweiler, M.C.; Richards, J.R.; Costa, D.L. Acute lung injury from intratracheal exposure to fugitive residual oil fly ash and its constituent metals in normo- and spontaneously hypertensive rats. Inhal. Toxicol. 2001, 13, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Hetland, R.B.; Ovrevik, J.; Sundfor, I.; Schwarze, P.E.; Lag, M. Different particle determinants induce apoptosis and cytokine release in primary alveolar macrophage cultures. Part. Fibre Toxicol. 2006, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Imrich, A.; Ning, Y.Y.; Koziel, H.; Coull, B.; Kobzik, L. Lipopolysaccharide priming amplifies lung macrophage tumor necrosis factor production in response to air particles. Toxicol. Appl. Pharmacol. 1999, 159, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xian, Y.F.; Loo, S.; Chan, W.Y.; Liu, L.; Lin, Z.X. Anti-atopic dermatitis effects of dictamni cortex: Studies on in vitro and in vivo experimental models. Phytomedicine 2021, 82, 153453. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Gallic acid-grafted chitooligosaccharides suppress antigen-induced allergic reactions in RBL-2H3 mast cells. Eur. J. Pharm. Sci. 2012, 47, 527–533. [Google Scholar] [CrossRef]
- Chen, G.; Li, K.K.; Fung, C.H.; Liu, C.L.; Wong, H.L.; Leung, P.C.; Ko, C.H. Er-Miao-San, a traditional herbal formula containing rhizoma atractylodis and cortex phellodendri inhibits inflammatory mediators in LPS-stimulated RAW264.7 macrophages through inhibition of NF-kappaB pathway and MAPKs activation. J. Ethnopharmacol. 2014, 154, 711–718. [Google Scholar] [CrossRef]
- Yamada, P.; Hatta, T.; Du, M.; Wakimizu, K.; Han, J.; Maki, T.; Isoda, H. Inflammatory and degranulation effect of yellow sand on RBL-2H3 cells in relation to chemical and biological constituents. Ecotoxicol. Environ. Saf. 2012, 84, 9–17. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Chae, S.; Park, J.S.; Youn, U.J.; Hyun, J.W. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J. Ethnopharmacol. 2010, 130, 299–306. [Google Scholar] [CrossRef]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Sun, B.; Nance, D.M. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J. Med. Food 2009, 12, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.S. Analysis of production and trade of Lycium chinense Mill. in Korea and China. J. Korean Soc. Int. Agric. 2012, 24, 425–428. [Google Scholar]
- Qin, W.T.; Wang, X.; Shen, W.C.; Sun, B.W. A novel role of kukoamine B: Inhibition of the inflammatory response in the livers of lipopolysaccharide-induced septic mice via its unique property of combining with lipopolysaccharide. Exp. Ther. Med. 2015, 9, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Suh, J.H.; Zheng, X.; Wang, Y.; Ho, C.T. Identification and Quantification of Potential Anti-inflammatory Hydroxycinnamic Acid Amides from Wolfberry. J. Agric. Food Chem. 2017, 65, 364–372. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Wang, D.; Tao, M.; Xu, W.; Olatunji, O.J. Anti-Inflammatory Activities of Kukoamine A From the Root Bark of Lycium chinense Miller. Nat. Prod. Commun. 2020, 15, 1934578X20912088. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, E.J.; Kim, E.O.; Choi, S.W. Study on the hypochlolesterolemic and antioxidative effects of tyramine derivatives from the root bark of Lycium chenese Miller. Nutr. Res. Pract. 2011, 5, 412–420. [Google Scholar] [CrossRef]
- Jeong, J.C.; Kim, S.J.; Kim, Y.K.; Kwon, C.H.; Kim, K.H. Lycii cortex radicis extract inhibits glioma tumor growth in vitro and in vivo through downregulation of the Akt/ERK pathway. Oncol. Rep. 2012, 27, 1467–1474. [Google Scholar]
- Kim, J.H.; Kim, E.Y.; Lee, B.; Min, J.H.; Song, D.U.; Lim, J.M.; Eom, J.W.; Yeom, M.; Jung, H.S.; Sohn, Y. The effects of Lycii Radicis Cortex on RANKL-induced osteoclast differentiation and activation in RAW 264.7 cells. Int. J. Mol. Med. 2016, 37, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Jung, H.J.; Woo, E.R. Antimicrobial property of (+)-lyoniresinol-3alpha-O-beta-D-glucopyranoside isolated from the root bark of Lycium chinense Miller against human pathogenic microorganisms. Arch. Pharm. Res. 2005, 28, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.; Kim, S.C.; Seo, J.Y.; Hong, S.B.; Park, Y.I. Immunostimulating activity of Lycium chinense Miller root extract through enhancing cytokine and chemokine production and phagocytic capacity of macrophages. J. Food Biochem. 2020, 44, e13215. [Google Scholar] [CrossRef]
- Ahn, M.; Park, J.S.; Chae, S.; Kim, S.; Moon, C.; Hyun, J.W.; Shin, T. Hepatoprotective effects of Lycium chinense Miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem. 2014, 116, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Liu, Y.; Tipoe, G.L.; Xing, F.; So, K.F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol Macromol. 2014, 69, 73–78. [Google Scholar] [CrossRef]
- Schroeder, J.T. Basophils: Emerging roles in the pathogenesis of allergic disease. Immunol. Rev. 2011, 242, 144–160. [Google Scholar] [CrossRef]
- Miyake, K.; Karasuyama, H. Emerging roles of basophils in allergic inflammation. Allergol. Int. 2017, 66, 382–391. [Google Scholar] [CrossRef]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharm. 2021, 138, 111534. [Google Scholar] [CrossRef]
- Moon, H.; White, A.C.; Borowsky, A.D. New insights into the functions of Cox-2 in skin and esophageal malignancies. Exp. Mol. Med. 2020, 52, 538–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, H.M.; Choi, S.H.; Kim, Y.; An, E.J.; Lee, S.H.; Kim, K.; Jung, H.J.; Jang, H.J. Effect of Rosa laevigata on PM10-induced inflammatory response of human lung epithelial cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 2893609. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Pandey, R.P.; Choi, J.W.; Sohng, J.K.; Choi, D.J.; Park, Y.I. Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. Int. Immunopharmacol. 2015, 25, 302–310. [Google Scholar] [CrossRef]
- Novak, N.; Kraft, S.; Bieber, T. IgE receptors. Curr. Opin. Immunol. 2001, 13, 721–726. [Google Scholar] [CrossRef]
- Korn, S.; Haasler, I.; Fliedner, F.; Becher, G.; Strohner, P.; Staatz, A.; Taube, C.; Buhl, R. Monitoring free serum IgE in severe asthma patients treated with omalizumab. Respir. Med. 2012, 106, 1494–1500. [Google Scholar] [CrossRef]
- Casale, T.B.; Costa, J.J.; Galli, S.J. TNF alpha is important in human lung allergic reactions. Am. J. Respir. Cell Mol. Biol. 1996, 15, 35–44. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, Z.; Harder, S.D.; Soukup, J.M. Apoptotic and inflammatory effects induced by different particles in human alveolar macrophages. Inhal. Toxicol. 2004, 16, 863–878. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H. Determination of Betaine in Lycii Cortex by Capillary Electrophoresis. IOP Conf. Ser. Mater. Sci. Eng. 2017, 274, 012073. [Google Scholar] [CrossRef]
- Han, E.H.; Kim, J.Y.; Kim, H.G.; Choi, J.H.; Im, J.H.; Woo, E.R.; Jeong, H.G. Dihydro-N-caffeoyltyramine down-regulates cyclooxygenase-2 expression by inhibiting the activities of C/EBP and AP-1 transcription factors. Food Chem. Toxicol. 2010, 48, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]








| Peaks | RT (minute) | m/z ([M + H]+) | Formula ([M + H]+) | Δppm | Compound |
|---|---|---|---|---|---|
| P1 | 5.14 | 531.3178 | C28 H43 O6 N4 | 0.11 | Kukoamine A or Kukoamine B |
| P2 | 8.37 | 329.1472 ([M + Na]+) | C16 H23 O4 N2 | 0.509 | 4-[4-(tert-Butoxycarbonyl)piperazin-1-yl]benzoic acid |
| P3 | 9.61 | 300.123 | C17 H18 O4 N | 0.018 | n-Caffeoyltyramine |
| P4 | 9.98 | 874.3726 | C42 H52 O12 N9 | −0.428 | Lyciumin a |
| P5 | 11.06 | 314.1385 | C18 H20 O4 N | −0.683 | Coumarin 314 |
| Genes | Primer Sequence a (F/R 5′ to 3′) | Location b | Size c | Cycling Parameters | Cycles |
|---|---|---|---|---|---|
| rat TNF-α | F: CGGAATTCGGCTCCCTCTCATCAGTTC R: GCTCTAGACCCTTGAAGAGAACCTGGG | F: 344 R: 573 | 230 | 94 °C 20 s, 60 °C 30 s, 72 °C 30 s | 35 |
| rat IL-4 | F: ACCTTGCTGTCACCCTGTTC R: TTGTGAGCGTGGACTCATTC | F: 17 R: 307 | 291 | 94 °C 20 s, 60 °C 30 s, 72 °C 30 s | 35 |
| rat IL-13 | F: GCTCTCGCTTGCCTTGGTGGTC R: CATCCGAGGCCTTTTGGTTAGAG | F: 31 R: 304 | 274 | 94 °C 20 s, 60 °C 30 s, 72 °C 30 s | 35 |
| rat COX2 | F: TGACTTTGGCAGGCTGGATT R: ACTGCACTTCTGGTACCGTG | F: 2945 R: 3064 | 120 | 94 °C 20 s, 55 °C 30 s, 72 °C 30 s | 35 |
| rat β-actin | F: AGCTATGAGCTGCCTGACG R: GGATGCCACAGGATTCCA | F: 793 R: 901 | 109 | 94 °C 20 s, 55 °C 30 s, 72 °C 30 s | 35 |
| mouse TNF-α | F: GGCAGGTCTACTTTGGAGTCATTGC R: ACATTCGAGGCTCCAGTG AATTCGG | F: 732 R: 1007 | 276 | 94 °C 30 s, 55 °C 30 s, 72 °C 40 s | 35 |
| mouse β-actin | F: TGCTGTCCCTGTATGCCTCT R: AGGTCTTTACGGATGTCAACG | F: 525 R: 967 | 443 | 94 °C 30 s, 55 °C 30 s, 72 °C 40 s | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ree, J.; Kim, H.J.; Kim, H.J.; Kim, W.J.; Choi, T.G.; Lee, S.; Hong, Y.K.; Hong, S.B.; Park, Y.I. Anti-Apoptotic and Anti-Inflammatory Effects of an Ethanolic Extract of Lycium chinense Root against Particulate Matter 10-Induced Cell Death and Inflammation in RBL-2H3 Basophil Cells and BALB/c Mice. Plants 2022, 11, 2485. https://doi.org/10.3390/plants11192485
Lee J, Ree J, Kim HJ, Kim HJ, Kim WJ, Choi TG, Lee S, Hong YK, Hong SB, Park YI. Anti-Apoptotic and Anti-Inflammatory Effects of an Ethanolic Extract of Lycium chinense Root against Particulate Matter 10-Induced Cell Death and Inflammation in RBL-2H3 Basophil Cells and BALB/c Mice. Plants. 2022; 11(19):2485. https://doi.org/10.3390/plants11192485
Chicago/Turabian StyleLee, Jisun, Jin Ree, Hyeon Jeong Kim, Hee Jin Kim, Woo Jung Kim, Tae Gyu Choi, Sanghyun Lee, Yun Ki Hong, Seong Bin Hong, and Yong Il Park. 2022. "Anti-Apoptotic and Anti-Inflammatory Effects of an Ethanolic Extract of Lycium chinense Root against Particulate Matter 10-Induced Cell Death and Inflammation in RBL-2H3 Basophil Cells and BALB/c Mice" Plants 11, no. 19: 2485. https://doi.org/10.3390/plants11192485
APA StyleLee, J., Ree, J., Kim, H. J., Kim, H. J., Kim, W. J., Choi, T. G., Lee, S., Hong, Y. K., Hong, S. B., & Park, Y. I. (2022). Anti-Apoptotic and Anti-Inflammatory Effects of an Ethanolic Extract of Lycium chinense Root against Particulate Matter 10-Induced Cell Death and Inflammation in RBL-2H3 Basophil Cells and BALB/c Mice. Plants, 11(19), 2485. https://doi.org/10.3390/plants11192485