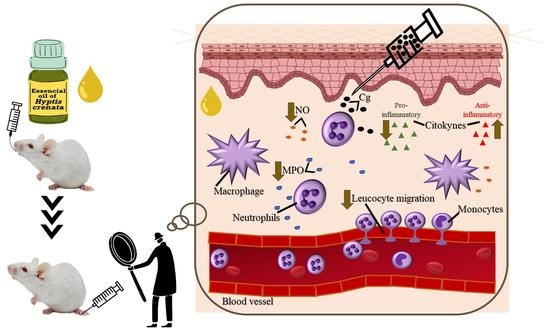
The Essential Oil of Hyptis crenata Inhibits the Increase in Secretion of Inflammatory Mediators
Abstract
:1. Introduction
2. Results
2.1. Effect of EOHc on Leucocyte Migration
2.2. Effect of EOHc on Myeloperoxidase Activity, Nitrite and Cytokines Dosage
2.2.1. Myeloperoxidase Activity and Nitrite Dosage
2.2.2. Cytokines
3. Discussion
4. Materials and Methods
4.1. Obtaining Essential Oil of Hyptis Crenata
4.2. Drugs and Solutions
4.3. Animals and Treatment
4.4. Peritonitis Induction
4.5. Myeloperoxidase Activity
4.6. Concentration of Nitrite (Griess Reaction)
4.7. Cytokines Quantification
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoghbi, M.D.G.B.; Andrade, E.H.A.; da Silva, M.H.L.; Maia, J.G.S.; Luz, A.I.R.; Da Silva, J.D. Chemical variation in the essential oils of Hyptis crenata Pohl ex Benth. Flavour Fragr. J. 2002, 17, 5–8. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Vieira, C.F.X.; Dos Santos, E.C.; Lima, G.C.; Aragão, K.K.V.; Vasconcelos, R.P.; Da Costa-Araújo, P.C.; Vasconcelos, Y.A.G.; De Oliveira, A.C.; De Oliveira, H.D. Gastroprotective effects of the essential oil of Hyptis crenata Pohl ex Benth. on gastric ulcer models. J. Ethnopharmacol. 2013, 149, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Scramin, S.; Saito, M.L.; Pott, A.; Marques, M.O.M. Volatile constituents of Hyptis crenata Pohl (Labiatae) native in Brazilian pantanal. J. Essent. Oil Res. 2000, 12, 99–101. [Google Scholar] [CrossRef]
- Di Stasi, L.C.; Oliveira, G.P.; Carvalhaes, M.A.; Queiroz-Junior, M.; Tien, O.S.; Kakinami, S.H.; Reis, M.S. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia 2002, 73, 69–91. [Google Scholar] [CrossRef]
- Neves, R.L.P.; Medeiros, A.P.R.; Lameira, O.A.; Rocha, T.T.; Ribeiro, F.N.S. Caracterização Fenológica da Espécie Hyptis crenata Pohl ex Benth No Campus da Embrapa Amazônia Oriental. In Congresso Brasileiro de Recursos Genéticos, Santos, Brazil (21 November 2014). 2014. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/112448/1/ResumoCBRG-106.pdf (accessed on 2 August 2022).
- World Checklist of Selected Plant Families. Hyptis crenata Pohl ex Benth., Labiat. Gen. Spec.: 93. 1833. Available online: https://wcsp.science.kew.org/namedetail.do?name_id=101705 (accessed on 2 August 2022).
- Jesus, N.Z.T.; Silva, J.C.; Silva, R.M.; Espinosa, M.M.; Martins, D.T.O. Levantamento etnobotânico de plantas popularmente utilizadas como antiúlceras e antiinflamatórias pela comunidade de Pirizal, Nossa Senhora do Livramento-MT, Brasil. Rev. Bras. Farmacogn. 2009, 19, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, M.A.; Magalhaes, R.M.; Torres, D.M.; Cavalcante, R.C.; Mota, F.S.; Oliveira-Coelho, E.M.; Moreira, H.P.; Lima, G.C.; Araujo, P.C.; Cardoso, J.H.; et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn. Mag. 2015, 11, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Lima, G.C.; Vasconcelos, Y.A.G.; De Santana Souza, M.T.; Oliveira, A.S.; Bomfim, R.R.; De Albuquerque-Júnior, R.L.C.; Camargo, E.A.; Portella, V.G.; Coelho-de-Souza, A.N.; Diniz, L.R.L. Hepatoprotective effect of essential oils from Hyptis crenata in sepsis-induced liver dysfunction. J. Med. Food 2018, 21, 709–715. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Alves-Soares, R.; Oliveira, H.D.; Gomes-Vasconcelos, Y.A.; Souza, P.J.C.; Santos-Nascimento, T.; Oliveira, K.A.; Diniz, L.R.L.; Guimarães-Pereira, J.; Leal-Cardoso, J.H. The essential oil of Hyptis crenata Pohl ex Benth. presents an antiedematogenic effect in mice. Braz. J. Med. Biol. Res. 2021, 54, 1–9. [Google Scholar] [CrossRef]
- Alves-Soares, R.; Gomes-Vasconcelos, Y.A.; Oliveira, K.A.; Diniz, L.R.L.; Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Leal-Cardoso, J.H.; Coelho-de-Souza, A.N. Evaluation of Oral Acute Tyoxicity of Essential Oil of Hyptis crenata (Pohl) Ex Benth in Mice. An. Acad. Cear. Ciênc. 2018, 2, 126–132. Available online: http://www.aceci.com.br/2019/03/12/anais-da-academia-cearense-de-ciencias-vol-02-n-02/ (accessed on 2 August 2022).
- Rebelo, M.M.; Silva, J.K.R.d.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant capacity and biological activity of essential oil and methanol extract of Hyptis crenata Pohl ex Benth. Rev. Bras. Farmacogn. 2009, 19, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Quintans, J.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes modulating cytokinesa—A review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Burrow, A.; Eccles, R.; Jones, A.S. The effects of camphor, eucalyptus and menthol vapour on nasal resistance to airflow and nasal sensation. Acta Otolaryngol. 1983, 96, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Li, X.-J.; Yang, Y.-J.; Li, Y.-S.; Zhang, W.K.; Tang, H.-B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Fang, C.; Tang, F. 1, 8-cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation 2014, 37, 566–572. [Google Scholar] [CrossRef]
- Yin, C.; Liu, B.; Wang, P.; Li, X.; Li, Y.; Zheng, X.; Tai, Y.; Wang, C.; Liu, B. Eucalyptol Alleviates Inflammation and Pain Responses in a Mouse Model of Gout Arthritis. Br. J. Pharmacol. 2019, 177, 566–572. [Google Scholar] [CrossRef]
- Linghu, K.-G.; Wu, G.-P.; Fu, L.-Y.; Yang, H.; Li, H.-Z.; Chen, Y.; Yu, H.; Tao, L.; Shen, X.-C. 1, 8-Cineole ameliorates LPS-induced vascular endothelium dysfunction in mice via PPAR-γ dependent regulation of NF-κB. Front. Pharmacol. 2019, 178, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Vasconcelos, Y.A.; Alves-Soares, R.; Silva-Junior, A.B.; Leal-Cardoso, J.H.; Coelho-De-Souza, A.N. Hyptis crenata—Popular Use, Obtained Compounds and Studied Biological Activities. An. Acad. Cear. Ciênc. 2019, 3, 39–43. Available online: http://www.aceci.com.br/2020/01/24/anais-da-academia-cearense-de-ciencias-volume-3-numero-1-e-2/ (accessed on 2 August 2022).
- Violante, I.; Garcez, W.S.; Barbosa, S.C.; Garcez, F.R. Chemical composition and biological activities of essential oil from Hyptis crenata growing in the Brazilian cerrado. Nat. Prod. Commun. 2012, 7, 1387–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, J.; Ferreira, A.E.; Silva, R.L.; Ulloa, L.; Grieco, P.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.d.Q.; Kanashiro, A. NDP-MSH inhibits neutrophil migration through nicotinic and adrenergic receptors in experimental peritonitis. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G. Cashew (Anacardium occidentale L.) nuts counteract oxidative stress and inflammation in an acute experimental model of Carrageenan-induced Paw edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Ross, R. The neutrophilic leukocyte in wound repair: A study with antineutrophil serum. J. Clin. Investig. 1972, 51, 2009–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Lee, W.L.; Harrison, R.E.; Grinstein, S. Phagocytosis by neutrophils. Microbes Infect. 2003, 5, 1299–1306. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Matsui, T.C.; Coura, G.M.; Melo, I.S.; Batista, C.R.; Augusto, P.S.A.; Godin, A.M.; Araújo, D.P.; César, I.C.; Ribeiro, L.S.; Souza, D.G. Nicorandil inhibits neutrophil recruitment in carrageenan-induced experimental pleurisy in mice. Eur. J. Pharmacol. 2015, 769, 306–312. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.; Assi, M.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.; Hezmee, M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell. Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, P.; Priebat, D.; Christensen, R.; Rothstein, G. Measurement of cutaneous inflammation: Estimation if neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizokami, S.S.; Hohmann, M.S.; Staurengo-Ferrari, L.; Carvalho, T.T.; Zarpelon, A.C.; Possebon, M.I.; de Souza, A.R.; Veneziani, R.C.; Arakawa, N.S.; Casagrande, R. Pimaradienoic acid inhibits carrageenan-induced inflammatory leukocyte recruitment and edema in mice: Inhibition of oxidative stress, nitric oxide and cytokine production. PLoS ONE 2016, 11, e0149656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Virág, L.; Jaén, R.I.; Regdon, Z.; Boscá, L.; Prieto, P. Self-defense of macrophages against oxidative injury: Fighting for their own survival. Redox Biol. 2019, 26, 101261. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef]
- Viaro, F.; Nobre, F.; Evora, P.R.B. Expression of nitric oxide synthases in the pathophysiology of cardiovascular diseases. Arq. Bras. Cardiol. 2000, 74, 380–393. [Google Scholar] [CrossRef] [Green Version]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Prado, W.A.; Schiavon, V.F.; Cunha, F.Q. Dual effect of local application of nitric oxide donors in a model of incision pain in rats. Eur. J. Pharmacol. 2002, 441, 57–65. [Google Scholar] [CrossRef]
- Adams, D.H.; Rlloyd, A. Chemokines: Leucocyte recruitment and activation cytokines. Lancet 1997, 349, 490–495. [Google Scholar] [CrossRef]
- Dinarello, C. Role of pro-and anti-inflammatory cytokines during inflammation: Experimental and clinical findings. J. Biol. Regul. Homeost. Agents 1997, 11, 91–103. Available online: https://europepmc.org/article/med/9498158 (accessed on 2 August 2022). [PubMed]
- Khan, H.A.; Khan, I.; Lee, Y.-k. Role of Immune Factors in Bioavailability and Toxicity of Carbon Nanomaterials. In Fullerens, Graphenes and Nanotubes; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 601–630. [Google Scholar] [CrossRef]
- Arango-Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]





| Experimental Model | Test | EC50 (mg/kg) |
|---|---|---|
| Peritonitis induced by carrageenan | Leucocytes migration | 36.88 |
| Peritonitis induced by carrageenan | Neutrophilic migration | 24.15 |
| Paw edema by carrageenan | MPO activity | 50.74 |
| Paw edema by carrageenan | Nitrite levels | 29.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Soares, R.; de Oliveira, H.D.; Campos, D.d.C.O.; Gomes-Vasconcelos, Y.d.A.; Ferreira-da-Silva, F.W.; Silva-Alves, K.S.; Coelho-de-Souza, L.N.; Diniz, L.R.L.; Leal-Cardoso, J.H.; Coelho-de-Souza, A.N. The Essential Oil of Hyptis crenata Inhibits the Increase in Secretion of Inflammatory Mediators. Plants 2022, 11, 3048. https://doi.org/10.3390/plants11223048
Alves-Soares R, de Oliveira HD, Campos DdCO, Gomes-Vasconcelos YdA, Ferreira-da-Silva FW, Silva-Alves KS, Coelho-de-Souza LN, Diniz LRL, Leal-Cardoso JH, Coelho-de-Souza AN. The Essential Oil of Hyptis crenata Inhibits the Increase in Secretion of Inflammatory Mediators. Plants. 2022; 11(22):3048. https://doi.org/10.3390/plants11223048
Chicago/Turabian StyleAlves-Soares, Rutyleia, Hermógenes David de Oliveira, Dyély de Carvalho Oliveira Campos, Yuri de Abreu Gomes-Vasconcelos, Francisco Walber Ferreira-da-Silva, Kerly Shamyra Silva-Alves, Lianna Noronha Coelho-de-Souza, Lúcio Ricardo Leite Diniz, José Henrique Leal-Cardoso, and Andrelina Noronha Coelho-de-Souza. 2022. "The Essential Oil of Hyptis crenata Inhibits the Increase in Secretion of Inflammatory Mediators" Plants 11, no. 22: 3048. https://doi.org/10.3390/plants11223048
APA StyleAlves-Soares, R., de Oliveira, H. D., Campos, D. d. C. O., Gomes-Vasconcelos, Y. d. A., Ferreira-da-Silva, F. W., Silva-Alves, K. S., Coelho-de-Souza, L. N., Diniz, L. R. L., Leal-Cardoso, J. H., & Coelho-de-Souza, A. N. (2022). The Essential Oil of Hyptis crenata Inhibits the Increase in Secretion of Inflammatory Mediators. Plants, 11(22), 3048. https://doi.org/10.3390/plants11223048