Saracura-Mirá, a Proposed Brazilian Amazonian Adaptogen from Ampelozizyphus amazonicus
Abstract
:1. Amazonian Medicinal Plants Used as Fortifiers and Tonics and the Relationship with Adaptogens—The Case of “Saracura-Mirá”
2. Botany, Management, Conservational and Vegetal Production Aspects
3. Ampelozizyphus amazonicus Ducke as an Adaptogen: Ethnobotanical and Ethnopharmacological Evidence
4. Pre-Clinical Pharmacological Studies with A. amazonicus
4.1. Pharmacological Evidence for A. amazonicus as an Adaptogen
4.2. Other Miscellaneous Pharmacological and Biological Activities
4.3. Safety and Toxicity Issues
5. Present Status of the Market, Supply Chains, Intellectual Property and Biotechnological Studies
6. Chemistry of Ampelozizyphus
7. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bungau, S.G.; Popa, V.-C. Between Religion and Science Some Aspects Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 24, 3–18. [Google Scholar]
- Oliveira, D.R.; Leitão, S.G. Fortifier, Tonic, and Rejuvenating Plants and the Adaptogen Concept. In Introduction to Ethnobiology, 1st ed.; Albuquerque, U.P., Nóbrega Alves, R., Eds.; Springer: Cham, Switzerland, 2016; pp. 151–162. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Leitão, G.G.; Castro, N.G.; Vieira, M.N.; ARQMO; Leitão, S.G. Ethnomedical Knowledge among the Quilombolas from the Amazon Region of Brazil with a Special Focus on Plants Used as Nervous System Tonics. In Medicinal Plants: Diversity and Drugs, 1st ed.; Raji, M., Rastrelli, L., Marinof, M., Martinez, J.L., Cordell, G., Eds.; CRC Press-Taylor & Francis Group: Enfield, NH, USA, 2012; pp. 142–178. [Google Scholar]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, A.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.; et al. Evolution of the Adaptogenic Concept from Traditional Use to Medical Systems: Pharmacology of Stress- and Aging-Related Diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Wagner, H. Plant Adaptogens III. Earlier and More Recent Aspects and Concepts on their Mode of Action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.R. Tonic, Fortifier and Aphrodisiac: Adaptogens in the Brazilian Folk Medicine. Rev. Bras. Farmacogn. 2011, 21, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, Z.; Bildziukevich, U.; Wimmerova, M.; Macurková, A.; Lovecká, P.; Wimmer, Z. Plant Adaptogens: Natural Medicaments for 21st Century? Chem. Select 2018, 3, 2196–2214. [Google Scholar] [CrossRef]
- Oliveira, D.R.; O’Dwyer, E.C.; Leitão, S.G.; Leitão, G.G.; ARQMO. Autorização de Acesso ao Conhecimento Tradicional Associado com Fins de Bioprospecção: O Caso da UFRJ e da Associação de Comunidades Quilombolas de Oriximiná—ARQMO. Rev. Fitos 2010, 5, 59–76. Available online: https://revistafitos.far.fiocruz.br/index.php/revista-fitos/article/view/104 (accessed on 10 October 2021).
- Slenes, R.W. Malungu, ngoma vem!”: África Coberta e Descoberta do Brasil. Revista USP 1992, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.S.D.; Muaze, M.A.F. Traditions in Movement: An Ethnohistory of the Health and Illness in the Valleys of the Acre and Purus Rivers, 1st ed.; Paralelo 15: Brasília, Brasil, 2002; p. 170. [Google Scholar]
- Maués, R.H. A Ilha Encantada—Medicina e Xamanismo numa Comunidade de Pescadores, 1st ed.; NAEA/UFPA: Belém, Brazil, 1990; p. 271. [Google Scholar]
- Buchillet, D. A Antropologia da Doença e os Sistemas Oficiais de Saúde. In Medicinas Tradicionais e Medicina Ocidental na Amazônia, 1st ed.; Buchillet, D., Ed.; CEJUP: Belém, Brazil, 1991; p. 487. [Google Scholar]
- Oliveira, D.R.; Costa, A.; Leitão, G.G.; Castro, N.G.; Santos, J.P.; Leitão, S.G. Estudo Etnofarmacognóstico da Saracuramirá (Ampelozizyphus amazonicus Ducke), uma Planta Medicinal Usada por Comunidades Quilombolas do Município de Oriximiná-PA, Brasil. Acta Amaz. 2011, 41, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.B. Lista de Espécies da Flora do Brasil, Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB207 (accessed on 31 October 2021).
- Lima, R.B.; Giuliette, A.M. Synonymies and Typification of the Rhamnaceae of Brazil. Acta Bot. Bras. 2014, 28, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Meier, W.; Berry, P.E. Ampelozizyphus guaquirensis (Rhamnaceae), a New Tree Species Endemic to the Venezuelan Coastal Cordillera. Brittonia 2008, 60, 131–135. [Google Scholar] [CrossRef]
- Lima, R.B. Flora da Reserva Ducke, Amazonas, Brasil: Rhamnaceae. Rodriguésia 2006, 57, 247–249. [Google Scholar] [CrossRef] [Green Version]
- Aymard, G.A.; Castro-Lima, F. A Second Tree Species of Ampelozizyphus (Rhamnaceae), from the Upper Cuyarí River Basin, Guianía (Colombia). Harv. Pap. Bot. 2015, 20, 161–166. [Google Scholar]
- Amaral, A.C.; Ferreira, J.L.P.; Moura, D.F.; Carvalho, J.; Ohana, D.T.; Echevarria, A.; Rosário, V.E.; Lopes, D.; Silva, J.R.A. Updated studies on Ampelozizyphus amazonicus, a medicinal plant used in the Amazonian Region. Phcog. Rev. 2008, 2, 308–316. [Google Scholar]
- Obase, D.J. Ecologia e Manejo do Cipó Saracuramirá, Ampelozizyphus amazonicus Ducke em um Assentamento Rural no Municipio de Presidente Figueiredo, AM. Master’s Thesis, Federal University of Amazonas (UFAM), Manaus, Brazil, 2006. Available online: https://repositorio.inpa.gov.br/handle/1/26298 (accessed on 10 October 2021).
- Siqueira, A.B. Ocorrencia de Populações Naturais de Ampelozizyphus amazonicus Ducke. e Piper peltatum L. ao Longo dos Rios Solimões e Amazonas e Estratégias de Conservação Ex Situ de Germoplasma por Técnicas In Vitro, Temperaturas Sub Zero e Criogênicas. Ph.D. Thesis, Federal University of Amazonas (UFAM), Manaus, Brazil, 2010. Available online: https://tede.ufam.edu.br/handle/tede/4393 (accessed on 10 October 2021).
- Viana-Junior, J. Propagação sexuada e assexuada de saracura-mirá (Ampelozizyphus amazonicus Ducke—Rhamnaceae), em Ambiente Natural e Viveiro, com Quatro Concentrações de Ácido Indol Butírico. Master’s Thesis, Federal University of Amazonas (UFAM), Manaus, Brazil, 2011. Available online: https://tede.ufam.edu.br/handle/tede/2739 (accessed on 10 October 2021).
- Van den Berg, M.E. Plantas Medicinais na Amazônia: Contribuição ao seu Conhecimento Sistemático, 1st ed.; Museu Paraense Emílio Goeldi: Belém, Brazil, 1982; 207p. [Google Scholar]
- Milliken, W. Traditional anti-malarial medicine in Roraima, Brazil. Econ. Bot. 1997, 51, 212–237. [Google Scholar]
- Milliken, W. Traditional Medicines Amongst Indigenous Groups in Roraima, Brazil: A Retrospective. Ethnoscientia 2021, 3, 116–139. [Google Scholar] [CrossRef]
- Santos, A.M.S.; Kahwage, C.C.; Ferreira, M.R.C.; Sampaio, N.A. Traditional Medicines in the Rio Negro Valley (Amazonas State, Brazil). Observations on the Ethnopharmacology and the Use of the Plant Saracuramirá (Ampelozizyphus amazonicus): Pharmacological Activity and/or Symbolic Efficacy. Bol. Mus. Para. Emílio Goeldi. Ciências Hum. 2005, 1, 137–147. [Google Scholar]
- Oliveira, D.R.; Leitão, G.G.; Coelho, T.S.; Silva, P.E.A.; Lourenço, M.C.S.; Leitão, S.G. Ethnopharmacological Versus Random Plant Selection Methods for the Evaluation of the Antimycobacterial Activity. Rev. Bras. Farmacogn. 2011, 21, 793–806. [Google Scholar]
- Oliveira, D.R.; Krettli, A.U.; Aguiar, A.C.C.; Leitão, G.G.; Vieira, M.N.; Martins, K.S.; Leitão, S.G. Ethnopharmacological Evaluation of Medicinal Plants Used Against Malaria by Quilombola Communities from Oriximiná, Brazil. J. Ethnopharmacol. 2015, 173, 424. [Google Scholar] [CrossRef] [Green Version]
- Le Cointe, P. Árvores e Plantas Úteis (Indígenas e Aclimadas): Nomes Vernáculos e Nomes Vulgares, Classificação Botanica, Habitat, Principais Aplicações e Propriedades, 2rd ed.; Cia. Ed. Nac.: São Paulo, Brasil, 1947; Volume 3, p. 506. [Google Scholar]
- Cid, P. Plantas Medicinais e Ervas Feiticeiras da Amazônia, 1st ed.; Atlantis: São Paulo, Brazil, 1978; p. 194. [Google Scholar]
- Ferrarini, S.A. Transertanismo: Sofrimento e Miséria do Nordestino na Amazônia, 1st ed.; Editora Vozes: Petrópolis, Brazil, 1979; 94p. [Google Scholar]
- Botelho, M.G.A.; Paulino-Filho, H.F.; Krettli, A.U. Quimioterapia Experimental Antimalárica Usando a Rhamnaceae Ampelozizyphus amazonicus Ducke, Vulgarmente Denominada “Cerveja-de-Índio” Contra o Plasmodium berghei. Oréades 1981, 8, 437–442. [Google Scholar]
- Biondo, M. Cartilha de Plantas Medicinais: Região Amazônica, 1st ed.; Gráfica LBA: Vitória, Brazil, 1987; p. 60. [Google Scholar]
- Rodrigues, R.M. A Flora da Amazonia, 1st ed.; CEJUP: Belém, Brazil, 1989; p. 462. [Google Scholar]
- Lorenz, S.S. Sateré-Mawé: Os Filhos do Guaraná, 1st ed.; Centro de Trabalho Indigenista: São Paulo, Brasil, 1992; p. 159. [Google Scholar]
- Ribeiro, J.E.L.; Hopkins, M.J.G.; Vicentini, A.; Sothers, C.A.; Costa, M.S.A.; Brito, J.M.; Souza, M.A.D.; Martins, L.H.P.; Lohmann, L.G.; Assunção, P.A.C.L.; et al. Flora da Reserva Ducke: Guia de Identificação das Plantas Vasculares de uma Floresta de Terra-Firme na Amazônia Central, 1st ed.; INPA: Manaus, Brazil, 1999; p. 816. [Google Scholar]
- Luz, F.J.F. Plantas medicinais de uso popular em Boa Vista, Roraima, Brasil. Hortaliça Bras. 2001, 19, 88–96. [Google Scholar]
- Krettli, A.U.; Andrade-Neto, V.F.; Brandão, M.G.L.; Ferrari, W.M.S. The Search for New Antimalarial Drugs from Plants Used to Treat Fever and Malaria or Plants Ramdomly Selected: A Review. Mem. Inst. Oswaldo Cruz 2001, 96, 1033–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, S.F.R.; Scarda, F.M. Plantas Medicinais: Etnobotânica na Várzea do Mamirauá, 1st ed.; SEBRAE: Manaus, Brazil, 2003; p. 218. [Google Scholar]
- Oliveira, D.R. Ethnobotanical Survey of Medicinal Plants Used in the City of Oriximiná (Pará State) with Ethnopharmacology Focus to the Lippia Genus. Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Borrás, M.R.L. Plantas da Amazônia: Medicinais ou Mágicas? Plantas Comercializadas no Mercado Municipal Adolpho Lisboa; Valer: Manaus, Brazil, 2003; 89p. [Google Scholar]
- Rodrigues, E. Plants and Animals Utilized as Medicines in the Jaú National Park (JNP), Brazilian Amazon. Phytother. Res. 2006, 20, 378. [Google Scholar] [PubMed]
- Rutter, R.A. Catálogo de Plantas Útiles de la Amazonia Peruana. Comunidades y Culturas Peruanas n° 22, 3rd ed.; Instituto Lingüístico de Verano: Lima, Peru, 2008; p. 12. Available online: https://www.sil.org/system/files/reapdata/15/20/84/152084859055909028131365439971855184702/ccp22.pdf (accessed on 10 October 2021).
- Trivellato, C. Plants Used for Treatment of Malaria and Related Disease in Indigenous Communities in the River Uaupés in São Gabriel da Cachoeira—AM. Master’s Thesis, Universidade Estadual Paulista Júlio de Mesquita Filho, Faculdade de Ciências Agronômicas, Botucatu, Brazil, 2015. Available online: http://hdl.handle.net/11449/134000 (accessed on 10 October 2021).
- Kffuri, C.W.; Lopes, M.A.; Ming, L.C.; Odonne, G.; Kinupp, V.F. Antimalarial Plants Used by Indigenous People of the Upper Rio Negro in Amazonas, Brazil. J. Ethnopharmacol. 2016, 178, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Tomchinsky, B.; Ming, L.C.; Kinupp, V.F.; Hidalgo, A.F.; Chaves, F.C.M. Ethnobotanical Study of Antimalarial Plants in the Middle Region of the Negro River, Amazonas, Brazil. Acta Amaz. 2017, 47, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Meneguelli, A.Z.; Camargo, E.E.S.; Buccini, D.F.; Roriz, B.C.; Cerqueira, G.R.; Moreno, S.E. Ethnopharmacological and botanical evaluation of medicinal plants used by Brazilian Amazon Indian community. Interações 2020, 21, 633–645. [Google Scholar]
- Hamdan, A.A. Mulheres Indígenas do Rio Negro Compartilham Conhecimentos de Remédios Tradicionais Contra a COVID-19. Available online: https://www.socioambiental.org/pt-br/noticias-socioambientais/mulheres-indigenas-do-rio-negro-compartilham-conhecimentos-de-remedios-tradicionais-contra-a-COVID-19 (accessed on 9 September 2020).
- Pontes, C.F. Manual de Sobrevivência na Selva, 1st ed.; IBGE: Rio de Janeiro, Brazil, 1993.
- MEB—Movimento de Educação de Base—Parintins-AM. In Popular Medicine Recipes; Universidade Federal do Amazonas: Manaus, Brazil, 1993; 26p. (In Portuguese)
- Storey, C.; Salem, J.I. Lay Use of Amazonian plants for the treatment of tuberculosis. Acta Amaz. 1997, 27, 175–185. [Google Scholar]
- Léda, P.H.O. Etnobotânica Aplicada às Plantas Medicinais como Subsídio para a Introdução de Espécies Nativas do Bioma Amazônia no Sistema Único de Saúde de Oriximiná—Pará, Brasil. Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, 2019. [Google Scholar]
- FRANCE IndígenasUsan Hierbas Medicinales Contra El Coronavirus en Brasil. Available online: https://www.france24.com/es/20200519-ind%C3%ADgenas-usan-hierbas-medicinales-contra-el-coronavirus-en-brasil (accessed on 19 May 2020).
- Brandão, M.G.L.; Lacaille-Dubois, M.A.; Teixeira, M.A.; Wagner, H. Triterpene Saponins from the Roots of Ampelozizyphus amazonicus. Phytochemistry 1992, 31, 352. [Google Scholar]
- Peçanha, L.M.T.; Fernandes, P.D.; Simen, T.J.M.; Oliveira, D.R.; Finotelli, P.V.; Pereira, M.V.A.; Barboza, F.F.; Almeida, T.S.; Carvalhal, S.; Leitao, G.G.; et al. Immunobiologic and Anti-inflammatory Properties of a Bark Extract from Ampelozizyphus amazonicus Ducke. BioMed Res. Int. 2013, 2013, 451679. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Neto, V.F.; Brandão, M.G.L.; Nogueira, F.; Rosário, V.E.; Krettli, A.U. Ampelozyziphus amazonicus Ducke (Rhamnaceae), a Medicinal Plant Used to Prevent Malaria in the Amazon Region, Hampers the Development of Plasmodium berghei Sporozoites. Int. J. Parasitol. 2008, 38, 1505. [Google Scholar] [CrossRef] [PubMed]
- Simen, T.J.M.; Finotelli, P.V.; Barboza, F.F.; Pereira, M.V.A.; Pierucci, A.P.T.R.; Moura, M.R.L.; Oliveira, D.R.; Abraçado, L.G.; Celano, R.; Figueiredo, F.S.; et al. Spray-dried Extract from the Amazonian Adaptogenic Plant Ampelozizyphus amazonicus Ducke (Saracura-mirá): Chemical Composition and Immunomodulatory Properties. Food Res. Int. 2016, 90, 100. [Google Scholar] [CrossRef]
- Song, X.; Hu, S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine 2009, 27, 4883–4890. [Google Scholar] [PubMed]
- Kaveri, S.V.; Silverman, G.J.; Bayry, J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J. Immunol. 2012, 188, 939–945. [Google Scholar]
- Do Carmo, D.F.M.; Amaral, A.C.F.; Machado, M.; Lopes, D.; Echevarria, A.; Rosário, V.E.; Silva, J.R.A. Evaluation of Antiplasmodial activity of extracts and constituents from Ampelozizyphus amazonicus. Pharmacogn. Mag. 2015, 11, S244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, L.R.L.; Santana, P.C.; Ribeiro, A.P.A.F.; Portella, V.G.; Pacheco, L.F.; Meyer, N.B.; Cesar, I.C.; Cosenza, G.P.; Brandão, M.G.L.; Vieira, M.A.R. Effect of triterpene saponins from roots of Ampelozizyphus amazonicus Ducke on diuresis in rats. J. Ethnopharmacol. 2009, 123, 275–279. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Portella, V.G.; Cardoso, F.M.; Souza, A.M.; Caruso-Neves, C.; Cassali, G.D.; Reis, A.M.; Brandão, M.G.L.; Vieira, M.A.R. The effect of saponins from Ampelozizyphus amazonicus Ducke on the renal Na+ pumps’ activities and urinary excretion of natriuretic peptides. BMC Complement. Altern. Med. 2012, 12, 40. Available online: http://www.biomedcentral.com/1472-6882/12/40 (accessed on 10 October 2021).
- Oliveira, D.R.; Oliveira, A.C.D.; Marques, L.C. The regulatory status of herbal medicines in Brazil: A comparison between the legislation and the pharmaceutical market (1995–2015). Vigil. Sanit. Debate 2016, 4, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Hoerner, W.S. Latin America and the Amazon Biological Resources. Research and Patents Concerning Amazon Herbal Medication. Master’s Thesis, Universidade de São Paulo, USP, São Paulo, Brazil. Available online: https://www.teses.usp.br/teses/disponiveis/84/84131/tde-10102012-171222/publico/Wagner_siloto_hoerner.pdf (accessed on 9 September 2020).
- Mendonça, S.C.; Simas, R.C.; Simas, D.L.R.; Leitão, S.G.; Leitão, G.G. Mass Spectrometry as a Tool for the Dereplication of Saponins from Ampelozizyphus amazonicus Ducke Bark and Wood. Phytochem. Anal. 2021, 32, 262. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/epdf/10.1002/pca.2972 (accessed on 10 October 2021).
- Nascimento, A.M.; Fonseca, T.S.; Campos, M.F.; Moreira, L.O.; Marques, C.A.; Tavares, E.S.; Mendonça, S.C.; Leitão, G.G.; Simas, R.C.; Leitão, S.G. Ziziphus joazeiro, a Saponin-rich Brazilian Medicinal Plant: Comparative Pharmacognostic Characterization of Bark and Leaves. Braz. J. Pharmacogn. 2020, 30, 756. [Google Scholar] [CrossRef]
- Medan, D.; Schirarend, C. Rhamnaceae. In The Families and Genera of Flowering Plants, 1st ed.; Kubitzki, K., Ed.; Springer: Berlin, Germany, 2004; Volume VI, p. 320. [Google Scholar] [CrossRef]
- Brandão, M.G.L.; Lacaille-Dubois, M.A.; Teixeira, M.A.; Wagner, H. A Dammarane-type Saponin from the Roots of Ampelozizyphus amazonicus. Phytochemistry 1993, 34, 1123. [Google Scholar] [CrossRef]
- De A Silva, J.R.; Correa, G.M.; Carvalho, R.; Costa, R.A.; Pinheiro, M.L.B.; Araújo, L.M.; Amaral, A.C.F. Analyses of Ampelozizyphus amazonicus, a Plant Used in Folk Medicine of the Amazon Region. Pharmacogn. Mag. 2009, 5, 75–80. Available online: https://www.researchgate.net/publication/287732834 (accessed on 10 October 2021).
- Rosas, V.L.; Cordeiro, M.S.C.; Campos, F.R.; Nascimento, S.K.R.; Januário, A.H.; FRança, S.C.; Nomizo, A.; Toldo, M.P.A.; Albuquerque, S.; Pereira, P.S. In vitro Evaluation of the Cytotoxic and Trypanocidal Activities of Ampelozizyphus amazonicus (Rhamnaceae). Braz. J. Med. Biol. Res. 2007, 40, 663–670. [Google Scholar] [PubMed] [Green Version]
- Figueiredo, F.S.; Celano, R.; Silva, D.S.; Costa, F.N.; Hewitson, P.; Ignatova, S.; Picinelli, A.R.; Rastrelli, L.; Leitão, S.G.; Leitão, G.G. Counter-current Chromatography Separation of Saponins by Skeleton Type from Ampelozizyphus amazonicus for Off-line Ultra-high-performance Liquid Chromatography/ High Resolution Accurate Mass Spectrometry Analysis and Characterization. J. Chromatogr. A 2017, 1481, 92. [Google Scholar] [CrossRef]






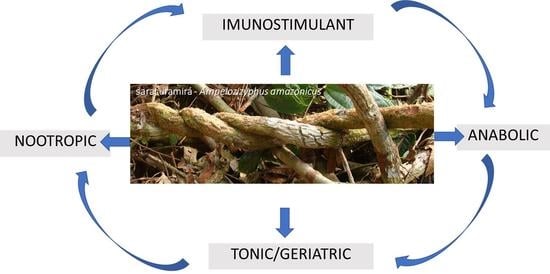
| Adaptogen Profile ** | Use Indications (Number of References that Cite Each Use Indication) | References |
|---|---|---|
| Immunostimulants (49) | treat malaria (16) *; prevent malaria (5) *; fever/febrile fever (5); chill (2); flu/cold (2); prevent disease (1); against infectious and parasitic diseases (1); “angry wound”/leishmaniasis (1); diarrhea (2); intestinal infection (1); sexually transmitted diseases (STDs) (1); verminosis (1); COVID-19 (1); inflammation in general (3); general pain/body pain (5); women’s inflammation (1); hemorrhoid (1) | [11,14,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] |
| Tonic and Geriatric Agents (32) | aphrodisiac (3); energetic/giving energy (2); tonic in general (1); antifatigue (1); stimulant (1); rejuvenating (1); revitalizing (2); indisposition (1); before working (1); appetizing (lack of appetite) (1); liver (or liver) disorders (5); gastrointestinal disorders (3); cholesterol (1); diabetes (2); kidney diseases (1); diuretic (1); purgative (1); well-being (1); insomnia/sleep disorders (2) | [14,24,27,40,41,42,43,50] |
| Nootropics (2) | nerve tonic (1); poor memory (1) | [3,28] |
| Anabolic Agents (3) | to become strong (2); fortifier (1) | [27,29,51] |
| Immunostimulant/Tonic and Geriatric Agents (18) | depurative (6); blood purifying (1); tuberculosis/severe cough/severe cough with blood (2); pneumonia (1); restoration of health (1); rheumatism (1); prostate inflammation (1); gastritis/stomach pain (2); intoxication (2); malaise due to alcohol abuse or fatty foods (1) | [26,27,29,35,41,44,52] |
| Anabolic/Tonic and Geriatric Agents (2) | anemia (2) | [3,41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, S.G.; Leitão, G.G.; de Oliveira, D.R. Saracura-Mirá, a Proposed Brazilian Amazonian Adaptogen from Ampelozizyphus amazonicus. Plants 2022, 11, 191. https://doi.org/10.3390/plants11020191
Leitão SG, Leitão GG, de Oliveira DR. Saracura-Mirá, a Proposed Brazilian Amazonian Adaptogen from Ampelozizyphus amazonicus. Plants. 2022; 11(2):191. https://doi.org/10.3390/plants11020191
Chicago/Turabian StyleLeitão, Suzana Guimarães, Gilda Guimarães Leitão, and Danilo Ribeiro de Oliveira. 2022. "Saracura-Mirá, a Proposed Brazilian Amazonian Adaptogen from Ampelozizyphus amazonicus" Plants 11, no. 2: 191. https://doi.org/10.3390/plants11020191
APA StyleLeitão, S. G., Leitão, G. G., & de Oliveira, D. R. (2022). Saracura-Mirá, a Proposed Brazilian Amazonian Adaptogen from Ampelozizyphus amazonicus. Plants, 11(2), 191. https://doi.org/10.3390/plants11020191