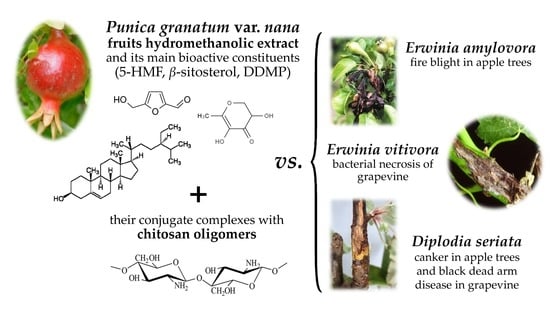
Dwarf Pomegranate (Punica granatum L. var. nana): Source of 5-HMF and Bioactive Compounds with Applications in the Protection of Woody Crops
Abstract
:1. Introduction
2. Results
2.1. Elementary Analysis
2.2. Thermal Analysis
2.3. Vibrational Characterization
2.4. Analysis of the Constituents of the Fruit Extract by GC−MS
2.5. Antimicrobial Activity
3. Discussion
3.1. Elemental Analysis
3.2. Thermal Analysis
3.3. Vibrational Characterization
3.4. Phytoconstituents Identified by GC−MS
3.5. Comparison of the Microbicidal Activity of the Extract
3.6. On the Synergistic Behavior after Conjugation with Chitosan Oligomers
4. Material and Methods
4.1. Plant Material
4.2. Reagents
4.3. Phytopathogen Isolates
4.4. Preparation of the Fruis Extract
4.5. Plant Biomass and Fruit Extract Physicochemical Characterization
4.6. In Vitro Antimicrobial Activity Assessment
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate Biology and Biotechnology: A Review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Moreno, P.M.; Valero, R.M. El Granado; Mundi Prensa Libros: Madrid, Spain, 1992; p. 164. [Google Scholar]
- Currò, S.; Caruso, M.; Distefano, G.; Gentile, A.; La Malfa, S. New Microsatellite loci for Pomegranate, Punica granatum (Lythraceae). Am. J. Bot. 2010, 97, e58–e60. [Google Scholar] [CrossRef]
- El-Moghazy, A.; Khalifa, A.; Bayoumi, S.; Sayed, H. Macro- and Micromorphology of the Leaves, Stem Bark and Flowers of Punica granatum L. var. nana Cultivated in Egypt. Bull. Pharm. Sci. Assiut 2015, 38, 99–125. [Google Scholar] [CrossRef] [Green Version]
- Heber, D.; Seeram, N.P.; Wyatt, H.; Henning, S.M.; Zhang, Y.; Ogden, L.G.; Dreher, M.; Hill, J.O. Safety and Antioxidant Activity of a Pomegranate Ellagitannin-Enriched Polyphenol Dietary Supplement in Overweight Individuals with Increased Waist Size. J. Agric. Food Chem. 2007, 55, 10050–10054. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial Potential of Pomegranate Peel: A Review. Int. J. Food Sci. Technol. 2018, 54, 959–965. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a Source of Bioactive Constituents: A Review on Their Characterization, Properties and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation. J. Food Qual. 2020, 2020, 8850339. [Google Scholar] [CrossRef]
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G. Biological Control of Tomato Bacterial Speck Using Punica granatum Fruit Peel Extract. Crop Prot. 2013, 46, 18–22. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Askarne, L.; Ezrari, S.; El Ghadaroui, L.; Tahiri, A.; Hrustić, J.; Amiri, S. Efficacy Assessment of Pomegranate Peel Aqueous Extract for Brown Rot (Monilinia spp.) Disease Control. Physiol. Mol. Plant Pathol. 2020, 110, 101482. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of Plant Extracts/es-590 Sential Oils against Three Plant Pathogenic Fungi and Mosquito Larvae: GC/MS Analysis of Bioactive Compounds. BioResources 2019, 14, 4489–4511. [Google Scholar] [CrossRef]
- Balah, M.; Nowra, A.A. Efficacy of Pomegranate (Punica granatum L.) and Henna (Lawsonia inermis L.) Natural Extracts to Control Some Plant Pathogens and Weeds. Egypt. J. Biol. Pest Control 2016, 26, 487. [Google Scholar]
- Pangallo, S.; Nicosia, M.G.L.D.; Agosteo, G.E.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Rapisarda, P.; Schena, L. Evaluation of a Pomegranate Peel Extract as an Alternative Means to Control Olive Anthracnose. Phytopathology 2017, 107, 1462–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tehranifar, A.; Selahvarzi, Y.; Kharrazi, M.; Bakhsh, V.J. High Potential of Agro-Industrial by-Products of Pomegranate (Punica granatum L.) as the Powerful Antifungal and Antioxidant Substances. Ind. Crop. Prod. 2011, 34, 1523–1527. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal Activity of Some Botanical Extracts on Fusarium Oxysporum. Open Life Sci. 2015, 10, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, A.M.; Khalifa, A.A.; Bayoumi, S.A.; Sayed, H.M. Chemical Constituents of Ornamental Pomegranate and Its Antioxidant and Anti-Inflammatory Activities in Comparison with Edible Pomegranate. J. Pharmacogn. Phytochem. 2016, 5, 88. [Google Scholar]
- Emam, A.; Ahmed, M.; Tammam, M.; Hala, A.; Zawam, S. Isolation and Structural Identification of Compounds with Antioxidant, Nematicidal and Fungicidal Activities from Punica granatum L. var. nana. Int. J. Sci. Eng. Res. 2015, 6, 1023–1040. [Google Scholar]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial Effect of the Tunisian Nana Variety Punica granatum L. Extracts against Salmonella enterica (Serovars Kentucky and Enteritidis) Isolated from Chicken Meat and Phenolic Composition of Its Peel Extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- El Deeb, K.S.; Eid, H.H.; Ali, Z.Y.; Shams, M.M.; Elfiky, A.M. Bioassay-Guided Fractionation and Identification of Antidiabetic Compounds from the Rind of Punica granatum var. nana. Nat. Prod. Res. 2019, 35, 2103–2106. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health. Scientific Opinion on the Pest Categorisation of Xylophilus ampelinus (Panagopoulos) Willems et al. EFSA J. 2014, 12, 3921. [Google Scholar] [CrossRef]
- Amri, Z.; Zaouay, F.; Lazreg-Aref, H.; Soltana, H.; Mneri, A.; Mars, M.; Hammami, M. Phytochemical Content, Fatty Acids Composition and Antioxidant Potential of Different Pomegranate Parts: Comparison between Edible and Non Edible Varieties Grown in Tunisia. Int. J. Biol. Macromol. 2017, 104, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ömeroğlu Ay, Ç.; Özcan, A.S.; Erdoğan, Y.; Özcan, A. Characterization of Punica granatum L. Peels and Quantitatively Determination of Its Biosorption Behavior towards Lead (II) Ions and Acid Blue 40. Colloids Surf. B Biointerfaces 2012, 100, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bretanha, M.S.; Rochefort, M.C.; Dotto, G.L.; Lima, E.C.; Dias, S.L.P.; Pavan, F.A. Punica granatum Husk (PGH), a Powdered Biowaste Material for the Adsorption of Methylene Blue Dye from Aqueous Solution. Desalination Water Treat. 2016, 57, 3194–3204. [Google Scholar] [CrossRef]
- Suryawanshi, P.C.; Kirtane, R.D.; Chaudhari, A.B.; Kothari, R.M. Conservation and Recycling of Pomegranate Seeds and Shells for Value Addition. J. Renew. Sustain. Energy 2009, 1, 013107. [Google Scholar] [CrossRef]
- Rowayshed, G.; Salama, A.; Abul-Fadl, M.; Akila-Hamza, S.; Emad, A.M. Nutritional and Chemical Evaluation for Pomegranate (Punica granatum L.) Fruit Peel and Seeds Powders by Products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- Abiola, T.; Falana, L.; Adediji, D. Proximate Composition, Phytochemical Analysis and in Vivo Antioxidant Activity of Pomegranate Seeds (Punica granatum) in Female Albino Mice. Biochem. Pharmacol. Open Access 2018, 7, 6. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish Biomass Wastes for Energy Use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef]
- International Organization for Standardization. Part 2: Graded wood pellets. In Solid Biofuels, Fuel Specifications and Classes; International Organization for Standardization: Geneva, Switzerland, 2014; Volume 17225, p. 9. [Google Scholar]
- ENplus. ENplus Handbook Version 3.0; European Biomass Association AEBIOM: Brussels, Belgium, 2015; p. 103. [Google Scholar]
- Selvaraj, S.; Rajkumar, P.; Thirunavukkarasu, K.; Gunasekaran, S.; Kumaresan, S. Vibrational (FT-IR and FT-Raman), Electronic (UV–Vis) and Quantum Chemical Investigations on Pyrogallol: A Study on Benzenetriol Dimers. Vib. Spectrosc 2018, 95, 16–22. [Google Scholar] [CrossRef]
- Lin, D.L.; Wang, S.M.; Wu, C.H.; Chen, B.G.; Liu, R.H. Chemical Derivatization for the Analysis of Drugs by GC-MS—A Conceptual Review. J. Food Drug Anal. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Vijayalakshmi, A. GC-MS Analysis of Phytochemical Constituents in Ethanolic Extract of Punica granatum Peel and Vitis vinifera Seeds. Int. J. Pharma Bio Sci. 2011, 2, 461–468. [Google Scholar]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and Distribution of Lignans in Punica granatum L. Fruit Endocarp, Pulp, Seeds, Wood Knots and Commercial Juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Mohamad, T.; Khalil, A. Effect of Pomegranate (Punica granatum L.) Fruits Peel on Some Phytopathogenic Fungi and Control of Tomato Damping-Off. Egypt. J. Phytopathol. 2014, 42, 171–186. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Thermal Stability of Anthocyanins and Colourless Phenolics in Pomegranate (Punica granatum L.) Juices and Model Solutions. Food Chem. 2013, 138, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.L.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A Kinetic Study on Microwave-Assisted Conversion of Cellulose and Lignocellulosic Waste into Hydroxymethylfurfural/Furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Prieto, P.; Martín-Ramos, P.; Hernández-Navarro, S.; Sánchez-Sastre, L.F.; Marcos-Robles, J.L.; Martín-Gil, J. Furfural, 5-HMF, Acid-Soluble Lignin and Sugar Contents in C. Ladanifer and E. Arborea Lignocellulosic Biomass Hydrolysates Obtained from Microwave-Assisted Treatments in Different Solvents. Biomass Bioenergy 2018, 119, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Nafea, E.; Moselhy, W.; Fawzy, A. Does the HMF Value Affect the Antibacterial Activity of the Bee Honey? Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 13–19. [Google Scholar] [CrossRef]
- Kaur, R.; Kaushal, S.; Sharma, P. Antimicrobial and Antioxidant Potential of Pomegranate (Punica granatum L.) Peel. Int. J. Chem. Stud. 2018, 6, 3441–3449. [Google Scholar]
- Čechovská, L.; Cejpek, K.; Konečný, M.; Velíšek, J. On the Role of 2,3-Dihydro-3,5-Dihydroxy-6-Methyl-(4H)-Pyran-4-One in Antioxidant Capacity of Prunes. Eur. Food Res. Technol. 2011, 233, 367–376. [Google Scholar] [CrossRef]
- Cynthia, F.I.; Hery, S.; Akhmad, D. Antibacterial and Antioxidant Activities of Pyrogallol and Synthetic Pyrogallol Dimer. Res. J. Chem. Environ. 2018, 22, 39–47. [Google Scholar]
- Sen, A.; Dhavan, P.; Shukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and Antimicrobial Activity of β-Sitosterol Isolated from Momordica charantia. Sci. Secur. J. Biotechnol. 2012, 1, 9–13. [Google Scholar]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. Induces G2/M Cell Cycle Arrest and Apoptosis through c-Myc Suppression in MCF-7 and A549 Cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.A.; El-Feky, G.S.; Elegami, H.M. Antibacterial Activity of Thirty Two Pomegranate (Punica granatum L.) Accessions Growing in Egypt Fruit Peels. World Appl. Sci. J. 2013, 21, 960–967. [Google Scholar]
- Mhaisgawali, M.; Gomashe, A.V.; Gogle, D.P.; Tumane, P.M.; Narwadiya, S.C. Antibacterial Investigation of Punica granatum (Pomegranate Rind) Extract against Different Plant Pathogenic Bacteria. Biosci. Biotechnol. Res. Asia 2010, 7, 377–382. [Google Scholar]
- Truchado, P.; Tomás-Barberán, F.A.; Larrosa, M.; Allende, A. Food Phytochemicals Act as Quorum sensing Inhibitors Reducing Production and/or Degrading Autoinducers of Yersinia enterocolitica and Erwinia carotovora. Food Control 2012, 24, 78–85. [Google Scholar] [CrossRef]
- Vlachou, P.; Holeba, M.; Termentzi, A.; Markellou, E.; Ntalli, N.; Skaltsounis, L.A.; Fokialakis, N. Exploitation of Agro-Industrial by-Products for Recovery of Bioactive Compounds with Applications in Pest Management. Planta Med. 2016, 82, P794. [Google Scholar] [CrossRef]
- Hussein, A.N.; Mohamed, R.Y.; Amein, T.A.M. Biological Control of Fire Blight Disease on Pear Caused by Erwinia amylovora in Erbil Province/Iraq. Tikrit J. Agric. Sci. 2019, 19, 65–71. [Google Scholar] [CrossRef]
- Khaleel, A.I.; Sijam, K.; Rashid, T.S.; Bin Ahmad, K. Phytochemical Determination and Antibacterial Activity of Punica granatum Peel Extracts against Plant Pathogenic Bacteria. Am. J. Plant Sci. 2016, 7, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Haynes, E.; Ramwell, C.; Griffiths, T.; Walker, D.; Smith, J. Report to Department for Environment, Food and Rural Affairs (Defra) & The Food Standards Agency (FSA). In Review of Antibiotic Use in Crops, Associated Risk of Antimicrobial Resistance and Research Gaps; Fera Science Ltd.: London, UK, 2020; p. 83. [Google Scholar]
- Matos, D.L.; Martes, I.N.; Silva, A.P.R.; Alaves, C.F.; David, G.Q.; Peres, W.M. Controle Alternativo de Lasiodiplodia theobromae com Óleos Vegetais. Cad. Agroecol. 2018, 13, 1–7. [Google Scholar]
- Lorenzetti, E.; Fujimoto, J.Y.H.; Faria, V.D.O.; Ritt, A.L.; Souza, D.H.G.; Tartaro, J.; Stangarlin, J.R. Avaliação in Vitro da Atividade Fungitóxica de Extratos de Plantas contra Diplodia Macrospora. Acta Iguazu 2020, 9, 113–122. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible Coatings Incorporating Pomegranate Peel Extract and Biocontrol Yeast to Reduce Penicillium digitatum Postharvest Decay of Oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.H.; Salem, M.F.; Mazrou, K.E.; El-Tras, W.F. Control of Citrus Molds Using Bioactive Coatings Incorporated with Fungal Chitosan/Plant Extracts Composite. J. Sci. Food Agric. 2016, 96, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and Antifungal Activity of Pomegranate Peel Extract and Its Use in Polysaccharide-Based Edible Coatings to Extend the Shelf-Life of Capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic Production of Fully Deacetylated Chitooligosaccharides and Their Neuroprotective and Anti-Inflammatory Properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal Agents Based on Chitosan Oligomers, ε-Polylysine and Streptomyces spp. Secondary Metabolites against Three Botryosphaeriaceae Species. Antibiotics 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talwalkar, A.T. IGT/DOE Coal-Conversion Systems Technical Data Book; Institute of Gas Technology: Chicago, IL, USA, 1981; p. 23. [Google Scholar]
- Wang, F.; Hu, L.J.; Zheng, Y.W.; Huang, Y.B.; Yang, X.Q.; Liu, C.; Kang, J.; Zheng, Z.F. Regulation for Optimal Liquid Products during Biomass Pyrolysis: A Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Beijing, China, 19–22 August 2016; Volume 40, p. 012047. [Google Scholar] [CrossRef] [Green Version]
- Nunn, S.; Nishikida, K. Advanced ATR Correction Algorithm-Application Note 50581; ThermoScientific: Madison, WI, USA, 2008; p. 4. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; p. 112. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST Technical Note on the EUCAST Definitive Document EDef 7.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [Green Version]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The Joint Action of Fungicides in Mixtures: Comparison of Two Methods for Synergy Calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]


| C (%) | H (%) | N (%) | O (by Difference, %) | C:N Ratio |
|---|---|---|---|---|
| 43.15 (42.7–43.4) | 6.41 (6.3–6.5) | 1.54 (1.3–1.6) | 48.9 | 28.1 |
| WaveNumber (cm−1) | Assignment |
|---|---|
| 3335 | bonded O−H stretching (cellulose, hemicellulose, lignin) |
| 2919 | −CH2 asymmetric stretching (alkyls) |
| 2850 | −CH2 symmetric stretching (cutin)/CH2−(C6)−bending (cellulose)/ C−H vibration of the aldehyde group (5-HMF) |
| 1730 | C = O stretching (alkyl esters) |
| 1624 | C = O stretching (hemicellulose, bonded ketones, …)/C−C-stretching |
| 1517 | aromatic skeletal (aromatic carotenoids) |
| 1444 | C−H deformation/C = C stretching of furan ring (furfural)/O−CH3 stretching |
| 1325 | CH in-plane bending (celluloses I and II) |
| 1226 | C−C−O asymmetric stretching (acetylated glucomannan)/ C−O and OH of the COOH/amide III |
| 1150 | C−O−C asymmetric stretching (celluloses I and II)/C−C in-plane (β−carotene) |
| 1101 | C−O−C stretching (pyranose ring skeleton in cellulose) |
| 1018 | C−H bending (carotenes)/polygalacturonic acid (pectin present in plant cuticles) |
| 913 | β−glycosidic linkage |
| 830 | CH2 rocking deformation/O−C=O in-plane deformation |
| Peak | Retention Time (min) | Area (%) | Assignment |
|---|---|---|---|
| 3 | 4.729 | 1.08 | propanoic acid, 2-oxo-, methyl ester |
| 8 | 5.752 | 1.06 | 2-furancarboxaldehyde, 5-methyl- (i.e., 5-methylfurfural) |
| 15 | 7.115 | 1.17 | hexanoic acid, 3-hydroxy-, methyl ester |
| 18 | 7.548 | 1.19 | 2,5-furandicarboxaldehyde (i.e., 5-formylfurfural) |
| 19 | 7.704 | 1.96 | 3,3-diacetyl-2,3,4,5-tetrahydro-2-oxofuran |
| 23 | 8.716 | 7.89 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (i.e., DDMP) |
| 24 | 8.741 | 1.81 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (i.e., DDMP) |
| 31 | 10.231 | 37.00 | 5-hydroxymethylfurfural (i.e., 5-HMF) |
| 32 | 10.314 | 2.28 | oxiniacic acid |
| 36 | 12.101 | 6.11 | 1,2,3-benzenetriol (i.e., pyrogallol) |
| 37 | 12.317 | 4.23 | hexanoic acid, 2-ethyl- |
| 41 | 14.700 | 1.47 | terpinen-4-ol |
| 44 | 18.391 | 1.19 | n-hexadecanoic acid (palmitic acid) |
| 53 | 24.559 | 1.06 | 9,12-octadecadienoic acid (Z,Z)- |
| 57 | 27.987 | 1.61 | D-α-tocopherol |
| 61 | 30.204 | 7.21 | β-/γ-sitosterol |
| Pathogen | Compound | Concentration (μg·mL−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 62.5 | 93.75 | 125 | 187.5 | 250 | 375 | 500 | 750 | 1000 | 1500 | ||
| E. amylovora | COS | + | + | + | + | + | + | + | + | + | − |
| P. granatum | + | + | + | + | + | + | + | + | + | − | |
| β-sitosterol | + | + | + | + | + | + | + | + | + | − | |
| 5-HMF | + | + | + | + | + | + | + | + | + | − | |
| DDMP | + | + | + | + | + | + | + | − | − | − | |
| COS−P. granatum | + | + | + | + | + | − | − | − | − | − | |
| COS−β-sitosterol | + | + | + | + | + | − | − | − | − | − | |
| COS−5-HMF | + | + | + | + | + | + | + | − | − | − | |
| COS−DDMP | + | + | + | + | + | − | − | − | − | − | |
| E. vitivora | COS | + | + | + | + | + | + | + | + | + | − |
| P. granatum | + | + | + | + | + | + | + | + | + | − | |
| β-sitosterol | + | + | + | + | + | + | + | + | + | − | |
| 5-HMF | + | + | + | + | + | + | + | + | − | − | |
| DDMP | + | + | + | + | + | + | − | − | − | − | |
| COS−P. granatum | + | + | + | + | − | − | − | − | − | − | |
| COS−β-sitosterol | + | + | + | + | + | + | − | − | − | − | |
| COS−5-HMF | + | + | + | + | + | + | − | − | − | − | |
| COS−DDMP | + | + | + | + | − | − | − | − | − | − | |
| EC | COS | P. granatum | COS− P. granatum | β−Sitosterol | COS− β-sitosterol | 5-HMF | COS− 5-HMF | DDMP | COS− DDMP |
|---|---|---|---|---|---|---|---|---|---|
| EC50 | 744.4 | 1656.4 | 623.0 | 82.0 | 51.0 | 442.6 | 212.8 | 317.8 | 158.0 |
| EC90 | 1179.9 | 4639.6 | 992.8 | 151.2 | 124.4 | 847.9 | 394.4 | 699.3 | 314.0 |
| SF | COS−P. granatum | COS−β-Sitosterol | COS−5-HMF | COS−DDMP |
|---|---|---|---|---|
| EC50 | 1.65 | 2.90 | 2.61 | 2.82 |
| EC90 | 1.89 | 2.15 | 2.50 | 2.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf Pomegranate (Punica granatum L. var. nana): Source of 5-HMF and Bioactive Compounds with Applications in the Protection of Woody Crops. Plants 2022, 11, 550. https://doi.org/10.3390/plants11040550
Sánchez-Hernández E, Buzón-Durán L, Cuchí-Oterino JA, Martín-Gil J, Lorenzo-Vidal B, Martín-Ramos P. Dwarf Pomegranate (Punica granatum L. var. nana): Source of 5-HMF and Bioactive Compounds with Applications in the Protection of Woody Crops. Plants. 2022; 11(4):550. https://doi.org/10.3390/plants11040550
Chicago/Turabian StyleSánchez-Hernández, Eva, Laura Buzón-Durán, José A. Cuchí-Oterino, Jesús Martín-Gil, Belén Lorenzo-Vidal, and Pablo Martín-Ramos. 2022. "Dwarf Pomegranate (Punica granatum L. var. nana): Source of 5-HMF and Bioactive Compounds with Applications in the Protection of Woody Crops" Plants 11, no. 4: 550. https://doi.org/10.3390/plants11040550
APA StyleSánchez-Hernández, E., Buzón-Durán, L., Cuchí-Oterino, J. A., Martín-Gil, J., Lorenzo-Vidal, B., & Martín-Ramos, P. (2022). Dwarf Pomegranate (Punica granatum L. var. nana): Source of 5-HMF and Bioactive Compounds with Applications in the Protection of Woody Crops. Plants, 11(4), 550. https://doi.org/10.3390/plants11040550