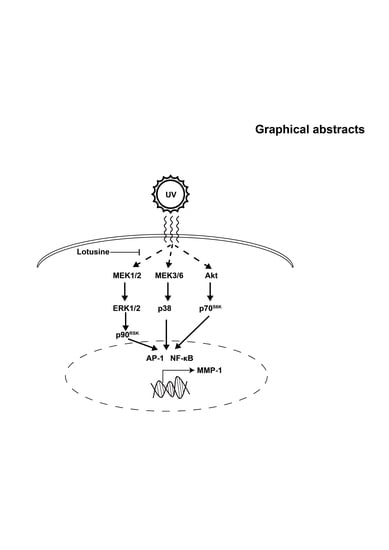
Inhibitory Effect of Lotusine on Solar UV-Induced Matrix Metalloproteinase-1 Expression
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Lotusine
2.2. Effects of Lotusine on sUV-Induced MMP-1 Expression in HaCaTCcells
2.3. Effects of Lotusine on sUV-Induced MMP-1 Promoter Activity by Suppressing AP-1 and NF-κB Transactivation
2.4. Lotusine Suppresses sUV-Induced MEK1/2-ERK1/2-p90RSK, MKK3/6-p38 and Akt-p70S6K Pathways in HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Viability
4.3. sUV Irradiation
4.4. Western Blotting
4.5. Gelatin Zymography
4.6. Luciferase Reporter Gene Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gilchrest, B.A. Photoaging. J. Invest. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todorova, K.; Mandinova, A. Novel approaches for managing aged skin and nonmelanoma skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 18–27. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F. Skin cancer and solar UV radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, D.X.; Kazimi, N.; Clydesdale, G.; Ananthaswamy, H.N.; Kripke, M.L.; Ullrich, S.E. Ultraviolet A radiation suppresses an established immune response: Implications for sunscreen design. J. Invest. Dermatol. 2001, 117, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Capell, B.C.; Parekh, V.; O’Day, C.; Atillasoy, C.; Bashir, H.M.; Yeh, C.; Shim, E.-H.; Prouty, S.M.; Dentchev, T.; et al. Whole-Exome and Transcriptome Analysis of UV-Exposed Epidermis and Carcinoma In Situ Reveals Early Drivers of Carcinogenesis. J. Invest. Dermatol. 2021, 141, 295–307.e213. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Lavrovsky, Y.; Chatterjee, B.; Clark, R.A.; Roy, A. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000, 35, 521–532. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, K.W. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018, 24, 1533–1550. [Google Scholar] [CrossRef]
- Meng, X.-L.; Chen, M.-L.; Chen, C.-L.; Gao, C.-C.; Li, C.; Wang, D.; Liu, H.-S.; Xu, C.-B. Bisbenzylisoquinoline alkaloids of lotus (Nelumbo nucifera Gaertn.) seed embryo inhibit lipopolysaccharide-induced macrophage activation via suppression of Ca2+-CaM/CaMKII pathway. Food Agric. Immunol. 2019, 30, 878–896. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kim, H.J.; Cho, S.K.; Kang, W.Y.; Baek, H.; Jeon, H.Y.; Kim, B.; Kim, D. Nelumbo nucifera extracts as whitening and anti-wrinkle cosmetic agent. Korean J. Chem. Eng. 2011, 28, 424–427. [Google Scholar] [CrossRef]
- Sridhar, K.; Bhat, R. Lotus-A potential nutraceutical source. J. Agric. Sci. Technol. 2007, 3, 143–155. [Google Scholar]
- Yang, T.H.; Chen, C.M. On the alkaloids of Nelumbo nucifera Gaertn. Studies on the alkaloids of loti embryo. J. Chin. Chem. Soc. 1970, 17, 235–242. [Google Scholar] [CrossRef]
- Sable, N.V.; Pagar, S.A. A review on Lotus: Use in herbal cosmetics. RJTCS 2013, 4, 81–83. [Google Scholar]
- Zhao, X.-l. Nelumbo nucifera Gaertn. 荷 (He, Lotus). In Dietary Chinese Herbs: Chemistry, Harmacology and Clinical Evidence; Springer: Vienna, Austria, 2015; pp. 731–739. [Google Scholar]
- Yang, J. NMR spectroscopic analysis of lotusine. Magn. Reson. Chem. 2005, 43, 184–185. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Richard, B.; Nuzillard, J.-M.; Zeches, M.; Le Men-Olivier, L. Two cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1993, 32, 1591–1594. [Google Scholar] [CrossRef]
- Harishkumar, R.; Selvaraj, C.I. Lotusine, an alkaloid from Nelumbo nucifera (Gaertn.), attenuates doxorubicin-induced toxicity in embryonically derived H9c2 cells. Vitro Cell Dev. Biol. Anim. 2020, 56, 367–377. [Google Scholar] [CrossRef]
- Le Croueour, G.; Thepenier, P.; Richard, B.; Petermann, C.; Ghedira, K.; Zeches-Hanrot, M. Lotusine G: A new cyclopeptide alkaloid from Zizyphus lotus. Fitoterapia 2002, 73, 63–68. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-A.; Yoo, D.-Y. Protective effect of Polygonum multiflorum on cell damage in UVB-irradiated HaCaT keratinocytes. J. Korean Obstet. Gynecol. 2011, 24, 31–49. [Google Scholar]
- Wu, X.; Huang, W.; Luo, G.; Alain, L.A. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling. Mol. Cell. Biochem. 2013, 384, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Mutreja, A.; Agarwal, M.; Kushwaha, S.; Chauhan, A. Effect of Nelumbo nucifera seeds on the reproductive organs of female rats. Int. J. Reprod. Biomed. 2008, 6, 7–11. [Google Scholar]
- Gruber, F.; Marchetti-Deschmann, M.; Kremslehner, C.; Schosserer, M. The Skin Epilipidome in Stress, Aging, and Inflammation. Front Endocrinol. 2020, 11, 607076. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food. Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int. J. Mol. Sci. 2015, 16, 68–90. [Google Scholar] [CrossRef]
- Kim, M.-K.; Shin, J.-M.; Eun, H.C.; Chung, J.H. The Role of p300 Histone Acetyltransferase in UV-Induced Histone Modifications and MMP-1 Gene Transcription. PLoS ONE 2009, 4, e4864. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, T.-K.; Roh, E.; Shin, H.-S.; Kim, J.-E. Inhibitory Effect of Lotusine on Solar UV-Induced Matrix Metalloproteinase-1 Expression. Plants 2022, 11, 773. https://doi.org/10.3390/plants11060773
Ryu T-K, Roh E, Shin H-S, Kim J-E. Inhibitory Effect of Lotusine on Solar UV-Induced Matrix Metalloproteinase-1 Expression. Plants. 2022; 11(6):773. https://doi.org/10.3390/plants11060773
Chicago/Turabian StyleRyu, Tae-Kyeong, Eunmiri Roh, Han-Seung Shin, and Jong-Eun Kim. 2022. "Inhibitory Effect of Lotusine on Solar UV-Induced Matrix Metalloproteinase-1 Expression" Plants 11, no. 6: 773. https://doi.org/10.3390/plants11060773
APA StyleRyu, T. -K., Roh, E., Shin, H. -S., & Kim, J. -E. (2022). Inhibitory Effect of Lotusine on Solar UV-Induced Matrix Metalloproteinase-1 Expression. Plants, 11(6), 773. https://doi.org/10.3390/plants11060773