Response of Pea Plants (Pisum sativum cv. Ran 1) to NaCl Treatment in Regard to Membrane Stability and Photosynthetic Activity
Abstract
:1. Introduction
2. Results
2.1. Salt-Induced Alterations in Water Status, Electrolyte Leakage and Lipid Peroxidation of Pea Plants
2.2. Pigment Content in the Leaves of Pea Plants after Salt Treatment
2.3. Oxygen Evolution of the Whole Leaves after the Treatment of Pea Plants with NaCl
2.4. Photochemical Activity of PSII, PSI and Oxygen Evolving Complex (OEC) Affected by the NaCl Treatment
2.5. Salt-Induced Energy Distribution between the Main Pigment–Protein Complexes (77K Fluorescence)
3. Discussion
3.1. Alterations in the RWC, Membrane Stability and Lipid Peroxidation
3.2. Pigment Content and Oxygen-Evolving Ability of the Leaves of Pea Plants
3.3. Photochemical Activity of PSII and PSI Affected by the Salt Application
3.4. Activity of the Oxygen-Evolving Complex Affected by Salt Stress
3.5. Salt-induced Alterations in Energy Transfer between the Main Pigment–Protein Complexes
4. Materials and Methods
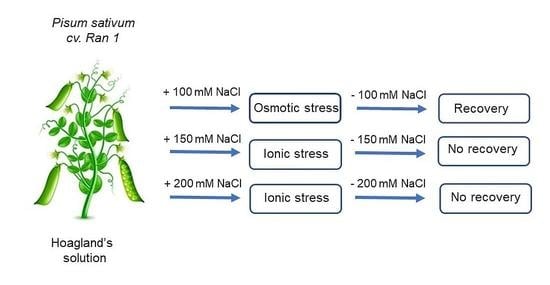
4.1. Plant Growth and Treatment
4.2. Determination of the Relative Water Content (RWC)
4.3. Electrolyte Leakage
4.4. Pigment Content of the Whole Leaves
4.5. Lipid Peroxidation
4.6. Oxygen-Evolving Activity of the Whole Leaves
4.7. Isolation of the Thylakoid Membranes
4.8. Photochemical Activities of PSII and PSI in the Isolated Thylakoid Membranes
4.9. Measurement of the Oxygen Flash Yields and Initial Oxygen Burst
4.10. 77K Fluorescence Measurements
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Tamanadar, E.; Moradi Pour, M.; Thakur, V.K. Salinity stress: Toward sustainable plant strategies and using plant growth-promoting rhizobacteria encapsulation for reducing it. Sustainability 2021, 13, 12758. [Google Scholar] [CrossRef]
- Datta, K.K.; Jong, C.D. Adverse effect of waterlogging and soil salinity on crop and land productivity in northwest region of Haryana. India. Agric. Water Manag. 2002, 57, 223–238. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Murata, N. Salt stress inhibits photosystem II and I in cynobacteria. Photosynth. Res. 2008, 98, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Rozema, J.; Flowers, T. Ecology: Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2017; pp. 24–63. [Google Scholar]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Zahra, N.; Hinai, M.S.A.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Ashraf, M.; Sultana, R. Combination effect of NaCl salinity and N-form on mineral composition of sunflower plants. Biol. Plant. 2000, 43, 615–619. [Google Scholar] [CrossRef]
- Winicov, I.; Seemann, J.R. Expression of genes for photosynthesis and the relationship to salt tolerance of alfalfa (Medicago sativa) cells. Plant Cell Physiol. 1990, 31, 1155–1161. [Google Scholar]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 6, 685–694. [Google Scholar] [CrossRef]
- Khavari-Nejad, R.A.; Mostofi, Y. Effects of NaCl on photosynthetic pigments, saccharides, and chloroplast ultrastructure in leaves of tomato cultivars. Photosynthetica 1998, 35, 151–154. [Google Scholar] [CrossRef]
- Raza, S.H.; Athar, H.R.; Ashraf, M.; Hameed, A. GB-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ. Exp. Bot. 2007, 60, 368–378. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Olmos, E.; Corpas, F.J.; Sevilla, F.; de1 Rio, L.A. Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci. 1995, 105, 151–167. [Google Scholar] [CrossRef]
- Monirifar, H.; Barghi, M. Identification and selection for salt tolerance in alfalfa (Medicago sativa L.) ecotypes via physiological traits. Not. Sci. Biol. 2009, 1, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Gomathi, R.; Rakkiyapan, P. Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int. J. Plant Physiol. Biochem. 2011, 3, 67–74. [Google Scholar]
- Ziaf, K.; Amjad, M.; Pervez, M.A.; Iqbal, Q.; Rajwana, I.A.; Ayyub, M. Evaluation of different growth and physiological traits as indices of salt tolerance in hot pepper (Capsicum annuum L.). Pak. J. Bot. 2009, 41, 1797–1809. [Google Scholar]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Pospisil, P. Production of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 2009, 1787, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Kan, X.; Ren, J.; Chen, T.; Cui, M.; Li, C.; Zhou, R.; Zhang, Y.; Lin, H.; Deng, D.; Yin, Z. Effects of salinity on photosynthesis in maize probed by prompt fluorescence, delayed fluorescence and P700 signals. Environ. Exp. Bot. 2017, 140, 56–64. [Google Scholar] [CrossRef]
- Zhang, H.H.; Xu, N.; Wu, X.; Wang, J.F.; Ma, S.; Li, X.; Sun, G. Effects of four types of sodium salt stress on plant growth and photosynthetic apparatus in sorghum leaves. J. Plant Interact. 2018, 13, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Aro, E.-M.; Tyystjarvi, E.; Kettunen, R. The rate constant of photoinhibition in vitro is dependent of the antenna size of Photosystem II but depends on temperature. Biochim. Biophys. Acta 1994, 1186, 177–185. [Google Scholar]
- Melis, A.; Guenther, G.E.; Morrissey, P.J.; Ghirardi, M.L. Photosystem II heterogeneity in chloroplasts. In Applications of Chlorophyll Fluorescence in Photosynthetic Research, Stress Physiology, Hydrobiology and Remote Sensing; Lichtenthaler, H.K., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 33–43. [Google Scholar]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 2005, 1706, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Koochak, H.; Puthiyaveetil, S.; Mullendore, D.L.; Li, M.; Kirchhof, H. The structural and functional domains of plant thylakoid membranes. Plant J. 2019, 97, 412–429. [Google Scholar] [CrossRef]
- Velitchkova, M.; Lazarova, D.; Popova, A.V. Response of isolated thylakoid membranes with altered fluidity to short heat stress. Physiol. Mol. Biol. Plants 2009, 15, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Velitchkova, M.; Borisova, P.; Vasilev, D.; Popova, A.V. Different impact of high light on the response and recovery of wild type and lut2 mutant of Arabidopsis thaliana at low temperature. Theor. Exp. Plant Physiol. 2021, 33, 95–111. [Google Scholar] [CrossRef]
- Lazarova, D.; Stanoeva, D.; Popova, A.; Vasilev, D.; Velitchkova, M. UV-B induced alteration of oxygen evolving reactions in pea thylakoid membranes as affected by scavengers of reactive oxygen species. Biol. Plant. 2014, 58, 319–327. [Google Scholar] [CrossRef]
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.N.; Iliadis, K. Differences in chemical composition of field pea (Pisum sativum) cultivars: Effects of cultivation area and year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Najafi, F.; Khavari-Nejad, R.A.; Rastgar-jazii, F.; Sticklen, M. Growth and some physiological attributes of pea (Pisum sativum L.) as affected by salinity. Pak. J. Biol. Sci. 2007, 10, 2752–2755. [Google Scholar]
- Shahid, M.A.; Pervez, M.A.; Balal, R.M.; Abbas, T.; Ayyub, C.M.; Mattson, N.S.; Riaz, A.; Iqbal, Z. Screening of pea (Pisum sativum L.) genotypes for salt tolerance based on early growth stage attributes and leaf inorganic osmolytes. Aust. J. Crop Sci. 2012, 6, 1324–1331. [Google Scholar]
- Hernandez, J.A.; Almansa, M.S. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant. 2002, 115, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.A.; Jimenez, A.; Mullineaux, P.; Sevilla, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Pandolfi, C.; Mancuso, S.; Shabala, S. Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ. Exp. Bot. 2012, 84, 44–51. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [Green Version]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2.−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Improvement in growth, chlorophyll pigments and photosynthetic performance in salt-stressed plants of sunflower (Helianthus annuus L.) by foliar application of 5-aminolevulinic acid. Agrochimica 2011, 55, 94–104. [Google Scholar]
- Velitchkova, M.; Popova, A.V. High light induced changes of 77 K fluorescence emission of pea thylakoid membranes with altered membrane fluidity. Bioelectrochemistry 2005, 67, 81–90. [Google Scholar] [CrossRef]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Adams, W.W., III; Zarter, C.R.; Mueh, K.E.; Amiard, V.; Demming-Adams, B. Energy dissipation and photoinhibition: A continuum of photoprotection. In Photoprotection, Photoinhibition, Gene Regulation, and Environment; Demming-Adams, B., Adams, W., Mattoo, A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 49–64. [Google Scholar]
- Faik, A.; Popova, A.V.; Velitchkova, M. Effects of long-term action of high temperature and high light on the activity and energy interaction of both photosystems in tomato plants. Photosynthetica 2016, 54, 611–619. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F.; Strasser, R.J. Functioning of photosystem I and II in pea leaves exposed to heat stress in the presence or absence of light. Planta 1991, 186, 88–98. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Gesheva, E.; Dimitrova, V.; Markovska, Y.; Doncheva, S.; Apostolova, E.L. Role of flavonoids and proline in the protection of photosynthetic apparatus in Paulownia under salt stress. S. Afr. J. Bot. 2021, 139, 246–253. [Google Scholar] [CrossRef]
- Hnilickova, H.; Hnilicka, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–431. [Google Scholar]
- Popova, A.V.; Dobrev, K.; Velitchkova, M.; Ivanov, A.G. Differential temperature effects on dissipation of excess light energy and energy partitioning in lut2 mutant of Arabodopsis thaliana under photoinhibitory conditions. Photosynth. Res. 2019, 139, 367–385. [Google Scholar] [CrossRef]
- Gerganova, M.T.; Faik, A.K.; Velitchkova, M.Y. Acquired tolerance of the photosynthetic apparatus to photoinhibition as a result of growing Solanum lycopersicum at moderately higher temperature and light intensity. Funct. Plant Biol. 2019, 46, 555–566. [Google Scholar] [CrossRef]
- Zeinalov, Y. An equipment for investigations of photosynthetic oxygen production reactions. Bulg. J. Plant Physiol. 2002, 28, 57–67. [Google Scholar]
- Kok, B.; Forbush, B.; McGloin, M. Co-operation of charges in photosynthetic O2 evolution. I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, A.V.; Borisova, P.; Vasilev, D. Response of Pea Plants (Pisum sativum cv. Ran 1) to NaCl Treatment in Regard to Membrane Stability and Photosynthetic Activity. Plants 2023, 12, 324. https://doi.org/10.3390/plants12020324
Popova AV, Borisova P, Vasilev D. Response of Pea Plants (Pisum sativum cv. Ran 1) to NaCl Treatment in Regard to Membrane Stability and Photosynthetic Activity. Plants. 2023; 12(2):324. https://doi.org/10.3390/plants12020324
Chicago/Turabian StylePopova, Antoaneta V., Preslava Borisova, and Dimitar Vasilev. 2023. "Response of Pea Plants (Pisum sativum cv. Ran 1) to NaCl Treatment in Regard to Membrane Stability and Photosynthetic Activity" Plants 12, no. 2: 324. https://doi.org/10.3390/plants12020324
APA StylePopova, A. V., Borisova, P., & Vasilev, D. (2023). Response of Pea Plants (Pisum sativum cv. Ran 1) to NaCl Treatment in Regard to Membrane Stability and Photosynthetic Activity. Plants, 12(2), 324. https://doi.org/10.3390/plants12020324