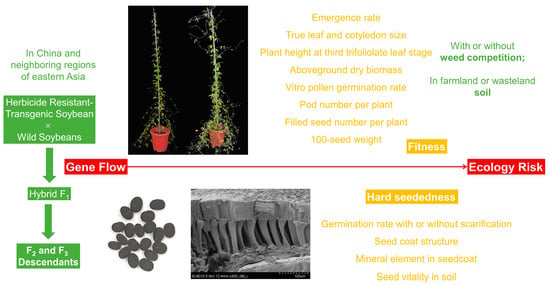
Fitness and Hard Seededness of F2 and F3 Descendants of Hybridization between Herbicide-Resistant Glycine max and G. soja
Abstract
:1. Introduction
2. Results
2.1. Emergence Rate
2.2. True Leaf and Cotyledon Size
2.3. Plant Height at Third Trifoliolate Leaf Stage
2.4. Aboveground Dry Biomass
2.5. Vitro Pollen Germination Rate
2.6. Pod and Filled Seed Number per Plant
2.7. 100-Seed Weight
2.8. Relative Composite Fitness
2.9. Hard Seededness and Germination Rate
2.10. Seed Coat Structure
2.11. Mineral Element in Seedcoat
2.12. Seed Vitality in Soil
3. Discussion
3.1. Fitness of F2, F3 Compared with Parents
3.2. Effects of Soil Nutrition and Competition on Fitness of F2, F3
3.3. Seed Coat Structure and Seed Dormancy
4. Materials and Methods
4.1. Seed Treatment and Seeding
4.2. Emergence Rate and Cotyledon, True Leaf Size
4.3. cp4-Epsps in Hybrids
4.4. Planting Conditions
4.5. Fitness Determination
4.6. Seed Hard Seededness Rate and Scarified Emergence Rate
4.7. Seed Coat Structure and Elemental Content
4.8. The Seed Vitality under Soil
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change; The International Service for the Acquisition of Agri-Biotech Applications (ISAAA): Ithaca, NY, USA, 2018; Volume 54. [Google Scholar]
- ISAAA. GM Approval Database. Available online: https://www.isaaa.org/gmapprovaldatabase/citations/default.asp (accessed on 17 August 2022).
- Wang, K.J.; Li, X.H. Interspecific gene flow and the origin of semi-wild soybean revealed by capturing the natural occurrence of introgression between wild and cultivated soybean populations. Plant Breed. 2011, 130, 117–127. [Google Scholar] [CrossRef]
- Kim, D.Y.; Heo, J.H.; Pack, I.S.; Park, J.-H.; Um, M.S.; Kim, H.J.; Park, K.W.; Nam, K.-H.; Oh, S.D.; Kim, J.K.; et al. Natural hybridization between transgenic and wild soybean genotypes. Plant Biotechnol. Rep. 2021, 15, 299–308. [Google Scholar] [CrossRef]
- Wang, K.J.; Li, X.H. Genetic diversity and gene flow dynamics revealed in the rare mixed populations of wild soybean (Glycine soja) and semi-wild type (Glycine gracilis) in China. Genet Resour. Crop Ev. 2013, 60, 2303–2318. [Google Scholar] [CrossRef]
- Kitamoto, N.; Kaga, A.; Kuroda, Y.; Ohsawa, R. A model to predict the frequency of integration of fitness-related QTLs from cultivated to wild soybean. Transgenic Res. 2012, 21, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mizuguti, A.; Yoshimura, Y.; Matsuo, K. Flowering phenologies and natural hybridization of genetically modified and wild soybeans under field conditions. Weed Biol. Manag. 2009, 9, 93–96. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamaguchi, H. Natural hybridization in wild soybean (Glycine max ssp. soja) by pollen flow from cultivated soybean (Glycine max ssp. max) in a designed population. Weed Biol. Manag. 2002, 2, 25–30. [Google Scholar] [CrossRef]
- Liu, B.; Xue, K.; Liu, L.-P.; Zhou, Y.; Han, J. Progress on the Gene Flow From Genetically Modified Soybeans to Wild Soybeans. J. Ecol. Rural Environ. 2020, 36, 833–841. [Google Scholar] [CrossRef]
- Kan, G.-Z. Fitness of Hybrids between Wild Soybeans(Glycine soja) and the Glyphosateresistant Transgenic Soybean (Glycine max). Soybean Sci. 2015, 34, 177–184. [Google Scholar]
- Kim, H.J.; Kim, D.Y.; Moon, Y.S.; Pack, I.S.; Park, K.W.; Chung, Y.S.; Kim, Y.J.; Nam, K.-H.; Kim, C.-G. Gene flow from herbicide resistant transgenic soybean to conventional soybean and wild soybean. Appl. Biol. Chem. 2019, 62, 54. [Google Scholar] [CrossRef]
- Jenczewski, E.; Ronfort, J.; Chevre, A.-M. Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environ. Biosaf. Res. 2003, 2, 9–24. [Google Scholar] [CrossRef]
- Wang, K.J.; Li, X.H. Phylogenetic relationships, interspecific hybridization and origin of some rare characters of wild soybean in the subgenus Glycine soja in China. Genet Resour. Crop Evol. 2012, 59, 1673–1685. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sheng, Z.W.; Hu, Y.Q.; Liu, Q.; Qiang, S.; Song, X.L.; Liu, B. Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean. Transgenic Res. 2021, 30, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C. Breaking physical dormancy in seeds-focussing on the lens. New Phytol. 2003, 158, 229–232. [Google Scholar] [CrossRef]
- Thiruppathi, D. Why so stubborn? MtKNOX4-regulated MtKCS12 manifests hardseededness. Plant Physiol. 2021, 186, 1367–1368. [Google Scholar] [CrossRef]
- Liu, A.; Ku, Y.S.; Contador, C.A.; Lam, H.M. The Impacts of Domestication and Agricultural Practices on Legume Nutrient Acquisition Through Symbiosis With Rhizobia and Arbuscular Mycorrhizal Fungi. Front. Genet. 2020, 11, 583954. [Google Scholar] [CrossRef]
- Liang, R.; Ji, X.; Sheng, Z.; Liu, J.; Qiang, S.; Song, X. Fitness and Rhizobacteria of F2, F3 Hybrids of Herbicide-Tolerant Transgenic Soybean and Wild Soybean. Plants 2022, 11, 3184. [Google Scholar] [CrossRef]
- Guan, Z.J.; Zhang, P.F.; Wei, W.; Mi, X.C.; Kang, D.M.; Liu, B. Performance of hybrid progeny formed between genetically modified herbicide-tolerant soybean and its wild ancestor. AoB Plants 2015, 7, plv121. [Google Scholar] [CrossRef] [PubMed]
- Yook, M.J.; Park, H.R.; Zhang, C.J.; Lim, S.H.; Jeong, S.C.; Chung, Y.S.; Kim, D.S. Environmental risk assessment of glufosinate-resistant soybean by pollen-mediated gene flow under field conditions in the region of the genetic origin. Sci. Total Environ. 2021, 762, 143073. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kaga, A.; Tomooka, N.; Yano, H.; Takada, Y.; Kato, S.; Vaughan, D. QTL affecting fitness of hybrids between wild and cultivated soybeans in experimental fields. Ecol. Evol. 2013, 3, 2150–2168. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Sheng, Z.-W.; Liu, J.-Y.; Liu, Q.; Qiang, S.; Song, X.-L.; Liu, B. Sexual compatibility of transgenic soybean and different wild soybean populations. J. Integr. Agr. 2022, 21, 36–48. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Ma, J. Genomic introgression through interspecific hybridization counteracts genetic bottleneck during soybean domestication. Genome Biol. 2019, 20, 22. [Google Scholar] [CrossRef]
- Kim, M.Y.; Van, K.; Kang, Y.J.; Kim, K.H.; Lee, S.H. Tracing soybean domestication history: From nucleotide to genome. Breed Sci. 2012, 61, 445–452. [Google Scholar] [CrossRef]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lozano, R.; Kim, J.H.; Bae, D.N.; Kim, S.T.; Park, J.H.; Choi, M.S.; Kim, J.; Ok, H.C.; Park, S.K.; et al. The patterns of deleterious mutations during the domestication of soybean. Nat. Commun. 2021, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.W.; Zhang, R.; Wu, Y.P.; Baskin, C.C. Seedling tolerance to cotyledon removal varies with seed size: A case of five legume species. Ecol. Evol. 2017, 7, 5948–5955. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Gong, Y.; Li, X.W.; Chen, P.; Ju, X.Y.; Zhang, C.M.; Yuan, B.; Lv, Z.P.; Xing, K.; Qin, S. Enhancement of growth and salt tolerance of tomato seedlings by a natural halotolerant actinobacterium Glutamicibacter halophytocola KLBMP 5180 isolated from a coastal halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]
- Clo, J.; Ronfort, J.; Gay, L. Fitness consequences of hybridization in a predominantly selfing species: Insights into the role of dominance and epistatic incompatibilities. Heredity 2021, 127, 393–400. [Google Scholar] [CrossRef]
- Winn, A.A.; Elle, E.; Kalisz, S.; Cheptou, P.O.; Eckert, C.G.; Goodwillie, C.; Johnston, M.O.; Moeller, D.A.; Ree, R.H.; Sargent, R.D.; et al. Analysis of Inbreeding Depression in Mixed-Mating Plants Provides Evidence for Selective Interference and Stable Mixed Mating. Evolution 2011, 65, 3339–3359. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Sun, J.; Dong, L.; Li, M.; Liu, Y.; Wang, J.; Zhang, X.; Li, D.; Sun, J.; et al. GmRAV confers ecological adaptation through photoperiod control of flowering time and maturity in soybean. Plant Physiol. 2021, 187, 361–377. [Google Scholar] [CrossRef]
- Singh, R.K.; Bhatia, V.S.; Bhat, K.V.; Mohapatra, T.; Singh, N.K.; Bansal, K.C.; Koundal, K.R. SSR and AFLP based genetic diversity of soybean germplasm differing in photoperiod sensitivity. Genet. Mol. Biol. 2010, 33, 319–324. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Ding, X.L.; Zhang, R.J.; Yang, Y.H.; Wei, B.G.; Yang, S.P.; Gai, J.Y. Transcriptome Analysis Reveals the Genes Related to Pollen Abortion in a Cytoplasmic Male-Sterile Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2022, 23, 12227. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Hymowitz, T. The Genomic Relationships among 6 Wild Perennial Species of the Genus Glycine Subgenus Glycine Willd. Theor. Appl. Genet. 1985, 71, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Nelson, R.L. Intersubgeneric hybridization between Glycine max and G-tomentella: Production of F-1, amphidiploid, BC1, BC2, BC3, and fertile soybean plants. Theor. Appl. Genet. 2015, 128, 1117–1136. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.M.; Hill, P.W.; Paterson, E.; Baggs, E.M.; Jones, D.L. Do plants use root-derived proteases to promote the uptake of soil organic nitrogen? Plant Soil 2020, 456, 355–367. [Google Scholar] [CrossRef]
- Otlewska, A.; Migliore, M.; Dybka-Stepien, K.; Manfredini, A.; Struszczyk-Swita, K.; Napoli, R.; Bialkowska, A.; Canfora, L.; Pinzari, F. When Salt Meddles Between Plant, Soil, and Microorganisms. Front. Plant Sci. 2020, 11, 553087. [Google Scholar] [CrossRef]
- Benezech, C.; Doudement, M.; Gourion, B. Legumes tolerance to rhizobia is not always observed and not always deserved. Cell Microbiol 2020, 22, e13124. [Google Scholar] [CrossRef]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial Associations with Legumes. Crit. Rev. Plant Sci. 2014, 34, 17–42. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.Y.; Selim, S.; Zinta, G.; Mousa, A.S.M.; Hozzein, W.N. Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism. Biomolecules 2020, 10, 1675. [Google Scholar] [CrossRef]
- Liu, J.; Yu, X.; Qin, Q.; Dinkins, R.D.; Zhu, H. The Impacts of Domestication and Breeding on Nitrogen Fixation Symbiosis in Legumes. Front. Genet. 2020, 11, 00973. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, J.; Zhao, D.L.; Meng, C.; Xu, Z.C.; Xie, Z.H.; Zhang, C.S. The Root Nodule Microbiome of Cultivated and Wild Halophytic Legumes Showed Similar Diversity but Distinct Community Structure in Yellow River Delta Saline Soils. Microorganisms 2020, 8, 207. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhao, Q.; He, R.; Zhang, W.; Zhang, H.J.; Wang, H.Y.; Ao, X.; Yao, X.D.; Xie, F.T. Rapid Effect of Enriched Nitrogen on Soybean Nitrogen Uptake, Distribution, and Assimilation During Early Flowering Stage. J. Soil Sci. Plant Nutr. 2022, 22, 3798–3810. [Google Scholar] [CrossRef]
- Oaks, A. Primary Nitrogen Assimilation in Higher-Plants and Its Regulation. Can. J. Bot. 1994, 72, 739–750. [Google Scholar] [CrossRef]
- Li, R.; Chen, H.; Yang, Z.; Yuan, S. Research status of soybean symbiosis nitrogen fixation. Oil Crop Sci. 2020, 5, 6–10. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Cao, F.; Kang, Y.; Chu, C.; Wu, S.; Li, J. Influence of 37 Years of Nitrogen and Phosphorus Fertilization on Composition of Rhizosphere Arbuscular Mycorrhizal Fungi Communities in Black Soil of Northeast China. Front. Microbiol. 2020, 11, 539669. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, H.; Shan, Y.Z.; Ma, H.Y.; Wang, H.Y.; Xie, F.T.; Ao, X. Physiological Response of Phosphorus-Efficient and Inefficient Soybean Genotypes under Phosphorus-Deficiency. Russ. J. Plant Physl+ 2020, 37, 175–184. [Google Scholar] [CrossRef]
- Ribet, J.; Drevon, J.J. Phosphorus Deficiency Increases the Acetylene-Induced Decline in Nitrogenase Activity in Soybean (Glycine-Max (L) Merr). J. Exp. Bot. 1995, 46, 1479–1486. [Google Scholar] [CrossRef]
- Mckenzie-Gopsill, A.G.; Lee, E.; Lukens, L.; Swanton, C.J. Rapid and early changes in morphology and gene expression in soya bean seedlings emerging in the presence of neighbouring weeds. Weed Res. 2016, 56, 267–273. [Google Scholar] [CrossRef]
- Wiles, L.J.; Wilkerson, G.G. Modeling Competition for Light between Soybean and Broadleaf Weeds. Agr. Syst. 1991, 35, 37–51. [Google Scholar] [CrossRef]
- Chetan, F.; Rusu, T.; Chetan, C.; Urda, C.; Rezi, R.; Simon, A.; Bogdan, I. Influence of Soil Tillage Systems on the Yield and Weeds Infestation in the Soybean Crop. Land 2022, 11, 1708. [Google Scholar] [CrossRef]
- Rockenbach, A.P.; Rizzardi, M.A. Competition at the soybean V6 stage affects root morphology and biochemical composition. Plant Biol. 2020, 22, 252–258. [Google Scholar] [CrossRef]
- Wagner, A. Competition for nutrients increases invasion resistance during assembly of microbial communities. Mol. Ecol. 2022, 31, 4188–4203. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Romero, E.; Aguirre-Noyola, J.L.; Taco-Taype, N.; Martinez-Romero, J.; Zuniga-Davila, D. Plant microbiota modified by plant domestication. Syst. Appl. Microbiol. 2020, 43, 126106. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Rondon, M.; Ito, O.; Ishikawa, T.; Rao, I.M.; Nakahara, K.; Lascano, C.; Berry, W.L. Biological nitrification inhibition (BNI)—Is it a widespread phenomenon? Plant Soil 2007, 294, 5–18. [Google Scholar] [CrossRef]
- Gong, T.; Xin, X.F. Phyllosphere microbiota: Community dynamics and its interaction with plant hosts. J. Integr. Plant Biol. 2020, 63, 297–304. [Google Scholar] [CrossRef]
- Gutierrez, A.; Grillo, M.A. Effects of Domestication on Plant-Microbiome Interactions. Plant Cell Physiol. 2022, 63, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The rhizosphere microbiome: Plant-microbial interactions for resource acquisition. J. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef]
- Xia, H.Y.; Wang, Z.G.; Zhao, J.H.; Sun, J.H.; Bao, X.G.; Christie, P.; Zhang, F.S.; Li, L. Contribution of interspecific interactions and phosphorus application to sustainable and productive intercropping systems. Field Crop Res. 2013, 154, 53–64. [Google Scholar] [CrossRef]
- Gijzen, M.; Weng, C.; Kuflu, K.; Woodrow, L.; Yu, K.; Poysa, V. Soybean seed lustre phenotype and surface protein cosegregate and map to linkage group E. Genome 2003, 46, 659–664. [Google Scholar] [CrossRef]
- Qutob, D.; Ma, F.; Peterson, C.A.; Bernards, M.A.; Gijzen, M. Structural and permeability properties of the soybean seed coat. Botany 2008, 86, 219–227. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, L.; Li, S.; Wang, W.; Ding, Y.; Swarm, S.A.; Li, L.; Wang, X.; Tang, X.; Zhang, Z.; et al. Elevation of soybean seed oil content through selection for seed coat shininess. Nat. Plants 2018, 4, 30–35. [Google Scholar] [CrossRef]
- Sun, L.; Miao, Z.; Cai, C.; Zhang, D.; Zhao, M.; Wu, Y.; Zhang, X.; Swarm, S.A.; Zhou, L.; Zhang, Z.J.; et al. GmHs1-1, encoding a calcineurin-like protein, controls hard-seededness in soybean. Nat. Genet 2015, 47, 939–943. [Google Scholar] [CrossRef]
- Satya Srii, V.; Nagarajappa, N.; Vasudevan, S.N. Is seed coat structure at fault for altered permeability and imbibition injury in artificially aged soybean seeds? Crop Sci. 2022, 62, 1573–1583. [Google Scholar] [CrossRef]
- Kuchlan, M.K.; Dadlani, M.; Samuel, D.V.K. Seed Coat Properties and Longevity of Soybean Seeds. J. New Seeds 2010, 11, 239–249. [Google Scholar] [CrossRef]
- Zablatzka, L.; Balarynova, J.; Klcova, B.; Kopecky, P.; Smykal, P. Anatomy and Histochemistry of Seed Coat Development of Wild (Pisum sativum subsp. elatius (M. Bieb.) Asch. et Graebn. and Domesticated Pea (Pisum sativum subsp. sativum L.). Int. J. Mol. Sci. 2021, 22, 4602. [Google Scholar] [CrossRef] [PubMed]
- Smykal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]













| Material | Hard Seededness Rate (%) | Germination Rate with Seed Scarification (%) |
|---|---|---|
| LNTL | 98.50 ± 0.96 ** | 94.44 ± 0.93 |
| LNTL F2 | 89.50 ± 0.96 | 93.86 ± 0.53 |
| LNTL | 100 | 98.00 ± 0.00 |
| LNTL F3 | 96.50 ± 0.02 | 94.50 ± 0.02 |
| Soils | Organic Matter g/kg | Total Nitrogen g/kg | Total Phosphorus g/kg | Total Potassium g/kg | Available Phosphorus mg/kg | Available Nitrogen mg/kg | |
|---|---|---|---|---|---|---|---|
| JLBC F2 | Wasteland soil | 2.79 ± 0.10 | 0.37 ± 0.01 | 0.56 ± 0.01 | 22.04 ± 0.46 | 22.39 ± 0.52 | 44.15 ± 0.2 |
| Farmland soil | 38.51 ± 0.35 * | 2.20 ± 0.03 * | 1.76 ± 0.01 * | 18.94 ± 0.19 | 47.81 ± 0.33 * | 163.74 ± 0.54 * | |
| JLBC F3 and LNTL F2 | Wasteland soil | 4.82 ± 0.22 | 0.27 ± 0.37 | 0.17 ± 0.11 | 9.79 ± 0.09 | 0.1 ± 0.03 | 10.71 ± 1.25 |
| Farmland soil | 9.74 ± 0.81 * | 0.37 ± 0.04 | 0.26 ± 0.12 * | 10.07 ± 0.10 | 1.68 ± 0.31 * | 23.59 ± 2.61 * | |
| LNTL F3 | Wasteland soil | 7.78 ± 0.40 | 0.72 ± 0.02 | 0.25 ± 0.01 | 20.94 ± 0.42 | 9.99 ± 0.86 | 51.91 ± 1.38 |
| Farmland soil | 11.19 ± 1.50 | 1.06 ± 0.11 | 0.36 ± 0.07 | 21.10 ± 0.48 | 28.21 ± 1.32 * | 145.41 ± 21.08 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Liu, J.-L.; Ji, X.-Q.; Olsen, K.M.; Qiang, S.; Song, X.-L. Fitness and Hard Seededness of F2 and F3 Descendants of Hybridization between Herbicide-Resistant Glycine max and G. soja. Plants 2023, 12, 3671. https://doi.org/10.3390/plants12213671
Liang R, Liu J-L, Ji X-Q, Olsen KM, Qiang S, Song X-L. Fitness and Hard Seededness of F2 and F3 Descendants of Hybridization between Herbicide-Resistant Glycine max and G. soja. Plants. 2023; 12(21):3671. https://doi.org/10.3390/plants12213671
Chicago/Turabian StyleLiang, Rong, Jia-Li Liu, Xue-Qin Ji, Kenneth M. Olsen, Sheng Qiang, and Xiao-Ling Song. 2023. "Fitness and Hard Seededness of F2 and F3 Descendants of Hybridization between Herbicide-Resistant Glycine max and G. soja" Plants 12, no. 21: 3671. https://doi.org/10.3390/plants12213671
APA StyleLiang, R., Liu, J. -L., Ji, X. -Q., Olsen, K. M., Qiang, S., & Song, X. -L. (2023). Fitness and Hard Seededness of F2 and F3 Descendants of Hybridization between Herbicide-Resistant Glycine max and G. soja. Plants, 12(21), 3671. https://doi.org/10.3390/plants12213671