Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities
Abstract
:1. Introduction
2. Nitrogen-Fixing Bacteria
Non-Symbiotic Nitrogen-Fixing Bacteria
3. Phosphate-Solubilizing Microorganisms
3.1. Solubilization of Inorganic Phosphorus Compounds
3.2. Mineralization of Organic Phosphorus Compounds
4. Siderophore-Secreting Microorganisms
4.1. Siderophores Produced by Soil Bacteria
4.2. Life Cycle of Siderophores
5. Isolation and Biochemical Analysis of Soil PGPB
5.1. Isolation of Nitrogen-Fixing Bacteria
5.2. Phenotypic Characteristics and Genotyping of Bacteria
5.3. Metabolic Characterization of Plant Growth Promotion
5.3.1. Solubilization of Phosphates and Potassium
5.3.2. Siderophore Production
5.3.3. Analysis of Phytohormone Production
5.4. Analysis of Antagonistic Activity against Phytopathogens and Competition between Strains
5.5. Analysis of Plant Growth Promotion
5.6. Bacterial Genes Promoting Plant Growth
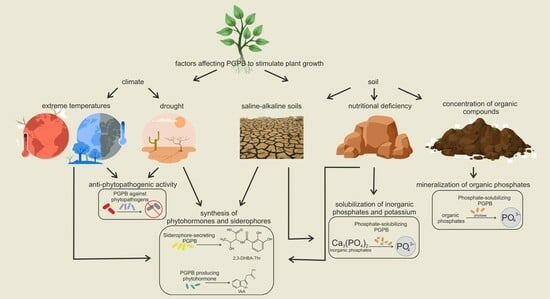
5.7. Environmental Factors That Can Affect PGPB and Increase or Decrease the Synthesis of Plant Growth-Promoting Substances
6. Consortia of Soil PGPB
Combined Application of Bacteria and Phosphate Fertilizers
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, A.R.L.E.; Ravichandran, K.; Antony, U. The impact of the Green Revolution on indigenous crops of India. J. Ethn. Foods 2019, 6, 8. [Google Scholar] [CrossRef]
- Fernández-Romero, M.; Parras-Alcántara, L.; Lozano-García, B.; Clark, J.; Collins, C. Soil Quality Assessment Based on Carbon Stratification Index in Different Olive Grove Management Practices in Mediterranean Areas. Catena 2016, 137, 449–458. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, P.; Mahapatra, S.C.; Mithun, S.K. Modelling Impacts of Chemical Fertilizer on Agricultural Production: A Case Study on Hooghly District, West Bengal, India. Model. Earth Syst. Environ. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Gupta, G.; Dhar, S.; Dass, A.; Sharma, V.K.; Shukla, L.; Singh, R.; Kumar, A.; Kumar, A.; Jinger, D.; Kumar, D.; et al. Assessment of Bio-Inoculants-Mediated Nutrient Management in Terms of Productivity, Profitability and Nutrient Harvest Index of Pigeon Pea–Wheat Cropping System in India. J. Plant Nutr. 2020, 43, 2911–2928. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Bacteria of Soil: Designing of Consortia Beneficial for Crop Production. Microorganisms 2023, 11, 2864. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Castaldi, S.; Petrillo, C.; Donadio, G.; Piaz, F.D.; Cimmino, A.; Masi, M.; Evidente, A.; Isticato, R. Plant Growth Promotion Function of Bacillus sp. Strains Isolated from Salt-Pan Rhizosphere and Their Biocontrol Potential against Macrophomina phaseolina. Int. J. Mol. Sci. 2021, 22, 3324. [Google Scholar] [CrossRef]
- Petrillo, C.; Castaldi, S.; Lanzilli, M.; Selci, M.; Cordone, A.; Giovannelli, D.; Isticato, R. Genomic and Physiological Characterization of Bacilli Isolated from Salt-Pans with Plant Growth Promoting Features. Front. Microbiol. 2021, 12, 715678. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Sheikh, I.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Amelioration of Drought Stress in Foxtail Millet (Setaria italica L.) by P-Solubilizing Drought-Tolerant Microbes with Multifarious Plant Growth Promoting Attributes. Environ. Sustain. 2020, 3, 23–34. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Lazali, M.; Bargaz, A. Examples of Belowground Mechanisms Enabling Legumes to Mitigate Phosphorus Deficiency. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability; Springer International Publishing: Cham, Switzerland, 2017; pp. 135–152. [Google Scholar]
- Roughley, R.J.; Gault, R.R.; Gemell, L.G.; Andrews, J.A.; Brockwell, J.; Dunn, B.W.; Griffiths, G.W.; Hartley, E.J.; Hebb, D.M.; Peoples, M.B.; et al. Autecology of Bradyrhizobium Japonicum in Soybean-Rice Rotations. Plant Soil 1995, 176, 7–14. [Google Scholar] [CrossRef]
- Ladha, J.K.; Tirol-Padre, A.; Reddy, C.K.; Cassman, K.G.; Verma, S.; Powlson, D.S.; van Kessel, C.; Richter, D.d.B.; Chakraborty, D.; Pathak, H. Global Nitrogen Budgets in Cereals: A 50-Year Assessment for Maize, Rice and Wheat Production Systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Roper, M.M.; Roget, D.K. Potential for non-symbiotic N2-fixation in different agroecological zones of southern Australia. Soil Res. 2006, 44, 343. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-Term Waterlogging has Long-Term Effects on the Growth and Physiology of Wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Ritika, B.; Utpal, D. Biofertilizer, a Way Towards Organic Agriculture: A Review. Afr. J. Microbiol. Res. 2014, 8, 2332–2343. [Google Scholar] [CrossRef]
- Barron, A.R.; Wurzburger, N.; Bellenger, J.P.; Wright, S.J.; Kraepiel, A.M.L.; Hedin, L.O. Molybdenum Limitation of Asymbiotic Nitrogen Fixation in Tropical Forest Soils. Nat. Geosci. 2009, 2, 42–45. [Google Scholar] [CrossRef]
- Ludden, P.W. Reversible ADP-Ribosylation as a Mechanism of Enzyme Regulation in Procaryotes. Mol. Cell. Biochem. 1994, 138, 123–129. [Google Scholar] [CrossRef]
- Robson, R.L.; Postgate, J.R. Oxygen and Hydrogen in Biological Nitrogen Fixation. Annu. Rev. Microbiol. 1980, 34, 183–207. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological Nitrogen Fixation and Prospects for Ecological Intensification in Cereal-Based Cropping Systems. Field Crop. Res. 2022, 283, 108541. [Google Scholar] [CrossRef] [PubMed]
- Eady, R.R. Structure−Function Relationships of Alternative Nitrogenases. Chem. Rev. 1996, 96, 3013–3030. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The Natural History of Nitrogen Fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Delavaux, C.S.; Smith-Ramesh, L.M.; Kuebbing, S.E. Beyond Nutrients: A Meta-Analysis of the Diverse Effects of Arbuscular Mycorrhizal Fungi on Plants and Soils. Ecology 2017, 98, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic BacteriumKushneriasp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D.L. Phosphate Reaction Dynamics in Soils and Soil Components: A Multiscale Approach. Adv. Agron. 2007, 94, 135–179. [Google Scholar]
- Seshachala, U.; Tallapragada, P. Phosphate Solubilizers from the Rhizospher of Piper nigrum L. in Karnataka, India. Chil. J. Agric. Res. 2012, 72, 397–403. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wang, J.; Ding, X. Phosphate Solubilization and Gene Expression of Phosphate-Solubilizing Bacterium Burkholderia multivorans WS-FJ9 under Different Levels of Soluble Phosphate. J. Microbiol. Biotechnol. 2017, 27, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Oubrie, A. Structure and Mechanism of Soluble Quinoprotein Glucose Dehydrogenase. EMBO J. 1999, 18, 5187–5194. [Google Scholar] [CrossRef] [PubMed]
- Lessie, T.G.; Phibbs, P.V. Alternative Pathways of Carbohydrate Utilization in Pseudomonads. Annu. Rev. Microbiol. 1984, 38, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Peix, A.; Mateos, P.F.; Rodriguez-Barrueco, C.; Martinez-Molina, E.; Velazquez, E. Growth promotion of common bean (Phaseolus vulgaris L.) by a strain of Burkholderia cepacia under growth chamber conditions. Soil Biol. Biochem. 2001, 33, 1927–1935. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2010; pp. 215–243. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Crowley, D.E.; Marschner, P.; Greiner, R.; Fernández, M.T.; Romero, D.; Menezes-Blackburn, D.; Mora, M.D.L.L. Identification of β-Propeller Phytase-Encoding Genes in Culturable paenibacillus and Bacillus spp. from the Rhizosphere of Pasture Plants on Volcanic Soils. FEMS Microbiol. Ecol. 2011, 75, 163–172. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Lidbury, I.D.E.A.; Murphy, A.R.J.; Scanlan, D.J.; Bending, G.D.; Jones, A.M.E.; Moore, J.D.; Goodall, A.; Hammond, J.P.; Wellington, E.M.H. Comparative genomic, proteomic and exoproteomic analyses of three Pseudomonas strains reveals novel insights into the phosphorus scavenging capabilities of soil bacteria. Environ. Microbiol. 2016, 18, 3535–3549. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Hernández, M.T.; Rengel, Z.; Marschner, P.; de la Luz Mora, M. Isolation of Culturable Phosphobacteria with Both Phytate-Mineralization and Phosphate-Solubilization Activity from the Rhizosphere of Plants Grown in a Volcanic Soil. Biol. Fertil. Soils 2008, 44, 1025–1034. [Google Scholar] [CrossRef]
- Rodríguez, H.; Rossolini, G.M.; Gonzalez, T.; Li, J.P.; Glick, B.R. Isolation of a Gene from Burkholderia cepacia IS-16 Encoding a Protein That Facilitates Phosphatase Activity. Curr. Microbiol. 2000, 40, 362–366. [Google Scholar] [CrossRef]
- Thaller, M.C.; Berlutti, F.; Schippa, S.; Iori, P.; Passariello, C.; Rossolini, G.M. Heterogeneous Patterns of Acid Phosphatases Containing Low-Molecular-Mass Polypeptides in Members of the Family Enterobacteriaceae. Int. J. Syst. Microbiol. 1995, 45, 255–261. [Google Scholar] [CrossRef]
- Ohtake, H.; Wu, H.; Imazu, K.; Anbe, Y.; Kato, J.; Kuroda, A. Bacterial Phosphonate Degradation, Phosphite Oxidation and Polyphosphate Accumulation. Resour. Conserv. Recycl. 1996, 18, 125–134. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hadobas, P.A. Soil Isolates of Pseudomonas spp. that Utilize Inositol Phosphates. Can. J. Microbiol. 1997, 43, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Heuck, C.; Smolka, G.; Whalen, E.D.; Frey, S.; Gundersen, P.; Moldan, F.; Fernandez, I.J.; Spohn, M. Effects of Long-Term Nitrogen Addition on Phosphorus Cycling in Organic Soil Horizons of Temperate Forests. Biogeochemistry 2018, 141, 167–181. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen Inputs Accelerate Phosphorus Cycling Rates Across a Wide Variety of Terrestrial Ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron Homeostasis in Host Defence and Inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An Archetype for Microbial Iron Transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef]
- Li, K.; Chen, W.-H.; Bruner, S.D. Microbial Siderophore-Based Iron Assimilation and Therapeutic Applications. BioMetals 2016, 29, 377–388. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron Metabolism in Pathogenic Bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Dertz, E.A.; Xu, J.; Stintzi, A.; Raymond, K.N. Bacillibactin-Mediated Iron Transport in Bacillus subtilis. J. Am. Chem. Soc. 2006, 128, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Scavino, A.F.; Pedraza, R.O. The Role of Siderophores in Plant Growth-Promoting Bacteria. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; pp. 265–285. [Google Scholar]
- Khasheii, B.; Mahmoodi, P.; Mohammadzadeh, A. Siderophores: Importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res. 2021, 250, 126790. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, J.C.; Robson, G.D.; Trinci, A.P.J.; Wiebe, M.G.; Livens, F.R.; Collison, D.; Taylor, R.J. Fungal siderophores: Structures, functions and applications. Mycol. Res. 2002, 106, 1123–1142. [Google Scholar] [CrossRef]
- Pecoraro, L.; Wang, X.; Shah, D.; Song, X.; Kumar, V.; Shakoor, A.; Tripathi, K.; Ramteke, P.W.; Rani, R. Biosynthesis Pathways, Transport Mechanisms and Biotechnological Applications of Fungal Siderophores. J. Fungi 2021, 8, 21. [Google Scholar] [CrossRef]
- Zabrieski, Z.; Morrell, E.; Hortin, J.; Dimkpa, C.; McLean, J.; Britt, D.; Anderson, A. Pesticidal Activity of Metal Oxide Nanoparticles on Plant Pathogenic Isolates of Pythium. Ecotoxicology 2015, 24, 1305–1314. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and Biology of Siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.M.; Challis, G.L. Recent Advances in Siderophore Biosynthesis. Curr. Opin. Chem. Biol. 2009, 13, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ustiatik, R.; Nuraini, Y.; Suharjono, S.; Handayanto, E. Siderophore Production of the Hg-Resistant Endophytic Bacteria Isolated from Local Grass in the Hg-Contaminated Soil. J. Ecol. Eng. 2021, 22, 129–138. [Google Scholar] [CrossRef]
- Butler, A.; Theisen, R.M. Iron(III)–Siderophore Coordination Chemistry: Reactivity of Marine Siderophores. Coord. Chem. Rev. 2010, 254, 288–296. [Google Scholar] [CrossRef]
- Byers, B.R.; Powell, M.V.; Lankford, C.E. Iron-chelating Hydroxamic Acid (Schizokinen) Active in Initiation of Cell Division in Bacillus megaterium. J. Bacteriol. 1967, 93, 286–294. [Google Scholar] [CrossRef]
- Storey, E.P.; Boghozian, R.; Little, J.L.; Lowman, D.W.; Chakraborty, R. Characterization of ‘Schizokinen’; a Dihydroxamate-type Siderophore Produced by Rhizobium Leguminosarum IARI 917. BioMetals 2006, 19, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Kamber, T.; Lansdell, T.A.; Stockwell, V.O.; Ishimaru, C.A.; Smits, T.H.M.; Duffy, B. Characterization of the Biosynthetic Operon for the Antibacterial Peptide Herbicolin in Pantoea vagans Biocontrol Strain C9-1 and Incidence in Pantoea Species. Appl. Environ. Microbiol. 2012, 78, 4412–4419. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Shoolery, J.N.; Schwyn, B.; Holden, I.; Neilands, J.B. Rhizobactin, a Structurally Novel Siderophore from Rhizobium Meliloti. J. Am. Chem. Soc. 1985, 107, 1739–1743. [Google Scholar] [CrossRef]
- Wright, W.; Little, J.; Liu, F.; Chakraborty, R. Isolation and structural identification of the trihydroxamate siderophore vicibactin and its degradative products from Rhizobium leguminosarum ATCC 14479 bv. trifolii. BioMetals 2013, 26, 271–283. [Google Scholar] [CrossRef]
- Aguirre-Noyola, J.L.; Rosenblueth, M.; Santiago-Martínez, M.G.; Martínez-Romero, E. Transcriptomic Responses of Rhizobium phaseoli to Root Exudates Reflect Its Capacity to Colonize Maize and Common Bean in an Intercropping System. Front. Microbiol. 2021, 12, 740818. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial Siderophore—A Boon to Agricultural Sciences. Biol. Control 2020, 144, 104214. [Google Scholar] [CrossRef]
- Mishra, A.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Ghosh, S. Iron Transport in Azospirillum brasilense: Role of the Siderophore Spirilobactin. Microbiology 1987, 133, 1759–1765. [Google Scholar] [CrossRef]
- Shah, S.; Karkhanis, V.; Desai, A. Isolation and Characterization of Siderophore, with Antimicrobial Activity, from Azospirillum Lipoferum M. Curr. Microbiol. 1992, 25, 347–351. [Google Scholar] [CrossRef]
- Tindale, A.E.; Mehrotra, M.; Ottem, D.; Page, W.J. Dual Regulation of Catecholate Siderophore Biosynthesis in Azotobacter Vinelandii by Iron and Oxidative Stress The GenBank Accession Number for the Sequence Reported in this Paper is AF238500. Microbiology 2000, 146, 1617–1626. [Google Scholar] [CrossRef]
- Kraepiel, A.M.L.; Bellenger, J.P.; Wichard, T.; Morel, F.M.M. Multiple Roles of siderophores in Free-Living Nitrogen-Fixing Bacteria. BioMetals 2009, 22, 573–581. [Google Scholar] [CrossRef]
- Eng-Wilmot, D.L.; Van der Helm, D. Molecular and Crystal Structure of the Linear Tricatechol Siderophore, Agrobactin. J. Am. Chem. Soc. 1980, 102, 7719–7725. [Google Scholar] [CrossRef]
- Ong, S.A.; Peterson, T.; Neilands, J.B. Agrobactin, a Siderophore from Agrobacterium Tumefaciens. J. Biol. Chem. 1979, 254, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Enzymatic Determination of Itoic Acid, a Bacillus Subtilis Siderophore, and 2,3-Dihydroxybenzoic Acid. Appl. Environ. Microbiol. 1993, 59, 2343–2345. [Google Scholar] [CrossRef]
- Wilson, M.K.; Abergel, R.J.; Raymond, K.N.; Arceneaux, J.E.L.; Byers, B.R. Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 2006, 348, 320–325. [Google Scholar] [CrossRef]
- Budzikiewicz, H. Secondary Metabolites from Fluorescent pseudomonads. FEMS Microbiol. Lett. 1993, 104, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Budzikiewicz, H. Siderophores of Fluorescent pseudomonads. Z. Für Naturforsch. C 1997, 52, 713–720. [Google Scholar] [CrossRef]
- Ringel, M.T.; Brüser, T. The Biosynthesis of Pyoverdines. Microb. Cell 2018, 5, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Philson, S.B.; Llinás, M. Siderochromes from Pseudomonas fluorescens. I. Isolation and characterization. J. Biol. Chem. 1982, 257, 8081–8085. [Google Scholar] [CrossRef]
- Youard, Z.A.; Mislin, G.L.A.; Majcherczyk, P.A.; Schalk, I.J.; Reimmann, C. Pseudomonas fluorescens CHA0 Produces Enantio-pyochelin, the Optical Antipode of the Pseudomonas aeruginosa Siderophore Pyochelin. J. Biol. Chem. 2007, 282, 35546–35553. [Google Scholar] [CrossRef]
- Mossialos, D.; Meyer, J.-M.; Budzikiewicz, H.; Wolff, U.; Koedam, N.; Baysse, C.; Anjaiah, V.; Cornelis, P. Quinolobactin, a New Siderophore of Pseudomonas fluorescens ATCC 17400, the Production of Which Is Repressed by the Cognate Pyoverdine. Appl. Environ. Microbiol. 2000, 66, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, S.; Budzikiewicz, H.; Schäfer, M.; Wathelet, B.; Cornelis, P. Ornicorrugatin, a New Siderophore from Pseudomonas fluorescens AF76. Z. Für Naturforsch. C 2008, 63, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Campestre, M.P.; Castagno, L.N.; Estrella, M.J.; Ruiz, O.A. Lotus Japonicus Plants of the Gifu B-129 Ecotype Subjected to Alkaline Stress Improve their Fe2+ bio-Availability through Inoculation with Pantoea Eucalypti M91. J. Plant Physiol. 2016, 192, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bulen, W.A.; LeComte, J.R. Isolation and Properties of a Yellow-Green Fluorescent Peptide from Azotobacter Medium. Biochem. Biophys. Res. Commun. 1962, 9, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Johri, B.N. Growth Promoting Influence of Siderophore-Producing Pseudomonas Strains GRP3A and PRS9 in Maize (Zea mays L.) under Iron Limiting Conditions. Microbiol. Res. 2003, 158, 243–248. [Google Scholar] [CrossRef]
- Ambrosi, C.; Leoni, L.; Putignani, L.; Orsi, N.; Visca, P. Pseudobactin Biogenesis in the Plant Growth-Promoting Rhizobacterium Pseudomonas Strain B10: Identification and Functional Analysis of the l -Ornithine N5-Oxygenase (psbA) Gene. J. Bacteriol. 2000, 182, 6233–6238. [Google Scholar] [CrossRef]
- Gründlinger, M.; Yasmin, S.; Lechner, B.E.; Geley, S.; Schrettl, M.; Hynes, M.; Haas, H. Fungal Siderophore Biosynthesis is Partially Localized in Peroxisomes. Mol. Microbiol. 2013, 88, 862–875. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Dick, R. Soil Health: The Theory of Everything (Terrestrial) or Just Another Buzzword? CSA News 2019, 63, 12–17. [Google Scholar] [CrossRef]
- Valenzuela-Aragon, B.; Parra-Cota, F.I.; Santoyo, G.; Arellano-Wattenbarger, G.L.; de los Santos-Villalobos, S. Plant-Assisted Selection: A Promising Alternative for in vivo Identification of Wheat (Triticum turgidum L. subsp. Durum) Growth Promoting Bacteria. Plant Soil 2018, 435, 367–384. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Lima Milani, K.M.; Azeredo Gonçalves, L.S.; Martinez de Oliveira, A.L. Diversity and Plant Growth-Promoting Functions of Diazotrophic/N-Scavenging Bacteria Isolated from the Soils and Rhizospheres of Two Species of Solanum. PLoS ONE 2020, 15, e0227422. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Urquieta, M.E.; Valenzuela-Ruíz, V.; Mitra, D.; Hyder, S.; Elsheery, N.I.; Das Mohapatra, P.K.; Parra-Cota, F.I.; Santos-de los Villalobos, S. Draft Genome Sequence of Priestia sp. Strain TSO9, a Plant Growth-Promoting Bacterium Associated with Wheat (Triticum turgidum subsp. durum) in the Yaqui Valley, Mexico. Plants 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-R.; Jiang, N. Extremely Rapid Extraction of DNA from Bacteria and Yeasts. Biotechnol. Lett. 2006, 28, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, J.; Guo, J. Two Phosphate- and Potassium-solubilizing Bacteria Isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of Potassium- and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Patel, N. Siderophores; Springer: New York, NY, USA, 2022; pp. 351–359. ISBN 978-1-0716-1724-3. [Google Scholar]
- Arnow, L.E. Proposed Chemical Mechanisms for The Production of Skin Erythema and Pigmentation By Radiant Energy. Science 1937, 86, 176. [Google Scholar] [CrossRef]
- Csáky, T.Z.; Hassel, O.; Rosenberg, T.; Lång (Loukamo), S.; Turunen, E.; Tuhkanen, A. On the Estimation of Bound Hydroxylamine in Biological Materials. Acta Chem. Scand. 1948, 2, 450–454. [Google Scholar] [CrossRef]
- Shenker, M.; Oliver, I.; Helmann, M.; Hadar, Y.; Chen, Y. Utilization by Tomatoes of Iron Mediated by a Siderophore Produced by Rhizopus arrhizus. J. Plant Nutr. 1992, 15, 2173–2182. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of Auxin Producing and Phosphate Solubilizing Bacteria on Mobility of Soil Phosphorus, Growth Rate, and P Acquisition by Wheat Plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Saraf, M.; Jha, C.K.; Patel, D. The Role of ACC Deaminase Producing PGPR in Sustainable Agriculture. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 365–385. [Google Scholar]
- Baudoin, E.; Lerner, A.; Mirza, M.S.; El Zemrany, H.; Prigent-Combaret, C.; Jurkevich, E.; Spaepen, S.; Vanderleyden, J.; Nazaret, S.; Okon, Y.; et al. Effects of Azospirillum brasilense with genetically modified auxin biosynthesis gene ipdC upon the diversity of the indigenous microbiota of the wheat rhizosphere. Res. Microbiol. 2010, 161, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Hu, X.; Huang, J. Origin of plant auxin biosynthesis. Trends Plant Sci. 2014, 19, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Espindula, E.; Sperb, E.R.; Moz, B.; Pankievicz, V.C.S.; Tuleski, T.R.; Tadra-Sfeir, M.Z.; Bonato, P.; Scheid, C.; Merib, J.; de Souza, E.M.; et al. Effects on gene expression during maize-Azospirillum interaction in the presence of a plant-specific inhibitor of indole-3-acetic acid production. Genet. Mol. Biol. 2023, 46, e20230100. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation of Indoleacetic Acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A Critical Examination of the Specificity of the Salkowski Reagent for Indolic Compounds Produced by Phytopathogenic Bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Santiago, C.D.; Yagi, S.; Ijima, M.; Nashimoto, T.; Sawada, M.; Ikeda, S.; Asano, K.; Orikasa, Y.; Ohwada, T. Bacterial Compatibility in Combined Inoculations Enhances the Growth of Potato Seedlings. Microbes Environ. 2017, 32, 14–23. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC Deaminase Producing PGPR from Rice Rhizosphere and Evaluating their Plant Growth Promoting Activity under Salt Stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Pranaw, K.; Pidlisnyuk, V.; Trögl, J.; Malinská, H. Bioprospecting of a Novel Plant Growth-Promoting Bacterium Bacillus Altitudinis KP-14 for Enhancing Miscanthus × giganteus Growth in Metals Contaminated Soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; Orozco-Mosqueda, M.d.C.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the Antifungal and Plant Growth-Promoting Effects of Diffusible and Volatile Organic Compounds Produced by Pseudomonas Fluorescens Strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate Availability Alters Architecture and Causes Changes in Hormone Sensitivity in the Arabidopsis Root System. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski-Dyé, F.; Lozano, L.; Acosta-Cruz, E.; Borland, S.; Drogue, B.; Prigent-Combaret, C.; Rouy, Z.; Barbe, V.; Herrera, A.M.; González, V.; et al. Genome Sequence of Azospirillum brasilense CBG497 and Comparative Analyses of Azospirillum Core and Accessory Genomes provide Insight into Niche Adaptation. Genes 2012, 3, 576–602. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Haidar, B.; Ferdous, M.; Fatema, B.; Ferdous, A.S.; Islam, M.R.; Khan, H. Population Diversity of Bacterial Endophytes from Jute (Corchorus olitorius) and Evaluation of Their Potential Role as Bioinoculants. Microbiol. Res. 2018, 208, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Hodgson, D.A.; Wentzel, A.; Nieselt, K.; Ellingsen, T.E.; Moore, J.; Morrissey, E.R.; Legaie, R.; The STREAM Consortium; Wohlleben, W.; et al. Metabolic Switches and Adaptations Deduced from the Proteomes of Streptomyces coelicolor Wild Type and phoP Mutant Grown in Batch Culture. Mol. Cell. Proteom. 2012, 11, M111.013797. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Archana, G.; Naresh Kumar, G. Metabolic Channeling of Glucose Towards Gluconate in Phosphate-Solubilizing Pseudomonas Aeruginosa P4 under Phosphorus Deficiency. Res. Microbiol. 2008, 159, 635–642. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wen, X. Effects of Soluble Phosphate on Phosphate-Solubilizing Characteristics and Expression of gcd Gene in Pseudomonas frederiksbergensis JW-SD2. Curr. Microbiol. 2016, 72, 198–206. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown Gold of Marginal Soil: Plant Growth Promoting Bacteria to Overcome Plant Abiotic Stress for Agriculture, Biofuels and Carbon Sequestration. Sci. Total. Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Weidner, S.; Friman, V.-P.; Xu, Y.-C.; Shen, Q.-R.; Jousset, A. Probiotic Pseudomonas Communities Enhance Plant Growth and Nutrient Assimilation via Diversity-Mediated Ecosystem Functioning. Soil Biol. Biochem. 2017, 113, 122–129. [Google Scholar] [CrossRef]
- Assainar, S.K.; Abbott, L.K.; Mickan, B.S.; Whiteley, A.S.; Siddique, K.H.M.; Solaiman, Z.M. Response of Wheat to a Multiple Species Microbial Inoculant Compared to Fertilizer Application. Front. Plant Sci. 2018, 9, 1601. [Google Scholar] [CrossRef] [PubMed]
- Agaras, B.C.; Scandiani, M.; Luque, A.; Fernández, L.; Farina, F.; Carmona, M.; Gally, M.; Romero, A.; Wall, L.; Valverde, C. Quantification of the Potential Biocontrol and Direct Plant Growth Promotion Abilities based on Multiple Biological Traits Distinguish Different Groups of Pseudomonas spp. Isolates. Biol. Control 2015, 90, 173–186. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, T.; Friman, V.-P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic Network Architecture of Root-Associated Bacterial Communities Determines Pathogen Invasion and Plant Health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wei, Z.; Friman, V.-P.; Gu, S.; Wang, X.; Eisenhauer, N.; Yang, T.; Ma, J.; Shen, Q.; Xu, Y.; et al. Probiotic Diversity Enhances Rhizosphere Microbiome Function and Plant Disease Suppression. mBio 2016, 7, e01790-16. [Google Scholar] [CrossRef] [PubMed]
- Kadmiri, I.M.; Chaouqui, L.; Azaroual, S.E.; Sijilmassi, B.; Yaakoubi, K.; Wahby, I. Phosphate-Solubilizing and Auxin-Producing Rhizobacteria Promote Plant Growth under Saline Conditions. Arab. J. Sci. Eng. 2018, 43, 3403–3415. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sau, G.B.; Sinha, S.; Mukherjee, S.K. Effect of Co-Inoculation of Plant Growth-Promoting Rhizobacteria on the Growth of Amaranth Plants. Arch. Agron. Soil Sci. 2012, 58, 1387–1397. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Bisht, S.; Singh, B.; Gulati, A.; Tewari, R. Enhanced Biomass and Steviol Glycosides in Stevia Rebaudiana Treated with Phosphate-Solubilizing Bacteria and Rock Phosphate. Plant Growth Regul. 2011, 65, 449–457. [Google Scholar] [CrossRef]
- Mahanta, D.; Rai, R.K.; Dhar, S.; Varghese, E.; Raja, A.; Purakayastha, T.J. Modification of Root Properties with Phosphate Solubilizing Bacteria and Arbuscular Mycorrhiza to Reduce Rock Phosphate Application in Soybean-Wheat Cropping System. Ecol. Eng. 2018, 111, 31–43. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-Ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Concomitant Rock Phosphate Dissolution and Lead Immobilization by Phosphate Solubilizing Bacteria (Enterobacter sp.). J. Environ. Manag. 2011, 92, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Abbasi, M.K.; Sultan, T. Isolation of Phosphate Solubilizing Bacteria from Maize Rhizosphere and Their Potential for Rock Phosphate Solubilization–Mineralization and Plant Growth Promotion. Geomicrobiol. J. 2017, 34, 81–95. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate Solubilizing Rhizobacteria Could Have a Stronger Influence on Wheat Root Traits and Aboveground Physiology Than Rhizosphere P Solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Ben Zineb, A.; Trabelsi, D.; Ayachi, I.; Barhoumi, F.; Aroca, R.; Mhamdi, R. Inoculation with Elite Strains of Phosphate-Solubilizing Bacteria Enhances the Effectiveness of Fertilization with Rock Phosphates. Geomicrobiol. J. 2020, 37, 22–30. [Google Scholar] [CrossRef]
- Tahir, M.; Khalid, U.; Ijaz, M.; Shah, G.M.; Naeem, M.A.; Shahid, M.; Mahmood, K.; Ahmad, N.; Kareem, F. Combined Application of Bio-Organic Phosphate and Phosphorus Solubilizing Bacteria (Bacillus strain MWT 14) Improve the Performance of Bread Wheat with low Fertilizer Input under an Arid Climate. Braz. J. Microbiol. 2018, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. https://doi.org/10.3390/plants12244074
Timofeeva AM, Galyamova MR, Sedykh SE. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants. 2023; 12(24):4074. https://doi.org/10.3390/plants12244074
Chicago/Turabian StyleTimofeeva, Anna M., Maria R. Galyamova, and Sergey E. Sedykh. 2023. "Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities" Plants 12, no. 24: 4074. https://doi.org/10.3390/plants12244074
APA StyleTimofeeva, A. M., Galyamova, M. R., & Sedykh, S. E. (2023). Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants, 12(24), 4074. https://doi.org/10.3390/plants12244074