Harnessing High Yield Potential in Wheat (Triticum aestivum L.) under Climate Change Scenario
Abstract
:1. Introduction
2. Results
2.1. Effect of Early Sowing and PGR on Grain Yield
2.2. Phenological, Physiological, and Yield Associated Traits
2.3. Correlation Analysis of Traits with Grain Yield
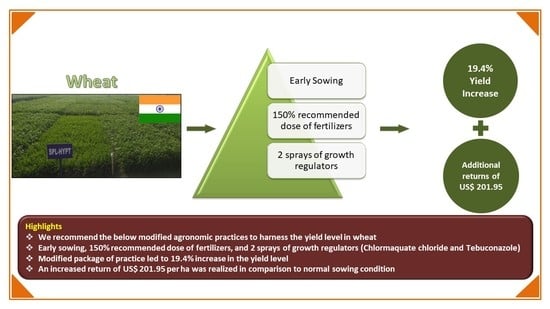
2.4. Partial Budgeting for HYPT
3. Discussion
4. Materials and Methods
4.1. Sowing Condition and Plant Material
4.2. Soil and Weather Condition of Study Locations
4.3. Agronomic Practices
4.4. Yield, Phenological, and Physiological Traits Measurement
4.5. Statistical Analysis
4.6. Partial Budgeting of HYPT Trial
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Rasheed, A.; Hickey, L.T.; He, Z. Fast-Forwarding Genetic Gain. Trends Plant Sci. 2018, 23, 184–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, R.A.; Connor, D.J. Issues for Cropping and Agricultural Science in the next 20 Years. Field Crops Res. 2018, 222, 121–142. [Google Scholar] [CrossRef]
- Fischer, R.A. Definitions and Determination of Crop Yield, Yield Gaps, and of Rates of Change. Field Crops Res. 2015, 182, 9–18. [Google Scholar] [CrossRef]
- Rajkumara, S. Lodging in Cereals—A Review. Agric. Rev. 2008, 29, 55–60. [Google Scholar]
- Singh, G. Director’s Report of AICRP on Wheat and Barley 2021–22; ICAR-IIWBR Karnal: Karnal, India, 2022; pp. 6–7. [Google Scholar]
- Khan, H.; Krishnappa, G.; Kumar, S.; Mishra, C.N.; Parkash, O.; Rathore, A.; Das, R.R.; Yadav, R.; Krishna, H.; Bishnoi, O.P.; et al. Genetic Gains in Grain Yield in Wheat (Triticum aestivum L.) Cultivars Developed from 1965 to 2020 for Irrigated Production Conditions of Northwestern Plains Zone of India. Cereal Res. Commun. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent Patterns of Crop Yield Growth and Stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [Green Version]
- Daloz, A.S.; Rydsaa, J.H.; Hodnebrog, Ø.; Sillmann, J.; van Oort, B.; Mohr, C.W.; Agrawal, M.; Emberson, L.; Stordal, F.; Zhang, T. Direct and Indirect Impacts of Climate Change on Wheat Yield in the Indo-Gangetic Plain in India. J. Agric. Food Res. 2021, 4, 100132. [Google Scholar] [CrossRef]
- Law, C.N.; Sutka, J.; Worland, A.J. A Genetic Study of Day-Length Response in Wheat. Heredity 1978, 41, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Worland, A.; Law, C. Genetic Analysis of Chromosome 2D of Wheat. I: The Location of Genes Affecting Height, Day-Lenght Insensitivity, Hybrid Dwarfism and Yellow-Rust Resistance. Z. Für Pflanz. 1986, 96, 331–345. [Google Scholar]
- Laurie, D.A. Comparative genetics of flowering time. Plant Mol. Biol. 1997, 35, 167–177. [Google Scholar] [CrossRef]
- Yan, L. The Wheat VRN2 Gene Is a Flowering Repressor Down-Regulated by Vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.K.; Mishra, B.; Chatrath, R.; Ortiz Ferrara, G.; Singh, R.P. Wheat Improvement in India: Present Status, Emerging Challenges and Future Prospects. Euphytica 2007, 157, 431–446. [Google Scholar] [CrossRef]
- Yadav, R.; Gaikwad, K.B.; Bhattacharyya, R. Breeding Wheat for Yield Maximization under Conservation Agriculture. Indian J. Genet. Plant Breed 2017, 77, 185. [Google Scholar] [CrossRef]
- Rajaram, S.; Van Ginkel, M.; Fischer, R. CIMMYT’s Wheat Breeding Mega-Environments (ME). In Proceedings of the 8th International Wheat Genetic Symposium; China Agricultural Scientech Press: Beijing, China, 1993; pp. 19–24. [Google Scholar]
- Sandhu, S.S.; Kaur, P.; Gill, K.K.; Vashisth, B.B. The Effect of Recent Climate Shifts on Optimal Sowing Windows for Wheat in Punjab, India. J. Water Clim. Chang. 2020, 11, 1177–1190. [Google Scholar] [CrossRef]
- van Rees, H.; McClelland, T.; Hochman, Z.; Carberry, P.; Hunt, J.; Huth, N.; Holzworth, D. Leading Farmers in South East Australia Have Closed the Exploitable Wheat Yield Gap: Prospects for Further Improvement. Field Crops Res. 2014, 164, 1–11. [Google Scholar] [CrossRef]
- Hunt, J.R.; Lilley, J.M.; Trevaskis, B.; Flohr, B.M.; Peake, A.; Fletcher, A.; Zwart, A.B.; Gobbett, D.; Kirkegaard, J.A. Early Sowing Systems Can Boost Australian Wheat Yields despite Recent Climate Change. Nat. Clim. Chang. 2019, 9, 244–247. [Google Scholar] [CrossRef]
- Naresh, K.S.; Aggarwal, P.; Swaroopa, R.D.; Saxena, R.; Chauhan, N.; Jain, S. Vulnerability of Wheat Production to Climate Change in India. Clim. Res. 2014, 59, 173–187. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, R.P.; Dreisigacker, S.; Röder, M.S.; Crossa, J.; Huerta-Espino, J.; Mondal, S.; Crespo-Herrera, L.; Singh, G.P.; Mishra, C.N.; et al. Juvenile Heat Tolerance in Wheat for Attaining Higher Grain Yield by Shifting to Early Sowing in October in South Asia. Genes 2021, 12, 1808. [Google Scholar] [CrossRef]
- Singh, G.; Chatrath, R.; Tyagi, B.; Singh, S.; Gupta, A.; Kumar, S.; Mishra, C.; Venkatesh, K.; Gupta, V.; Singh, C.; et al. Progress Report of AICRP on Wheat and Barley 2018–2019, Crop Improvement; ICAR-IIWBR Karnal: Karnal, India, 2019; pp. 6–7. [Google Scholar]
- Newport, D.; Lobell, D.B.; Balwinder-Singh; Srivastava, A.K.; Rao, P.; Umashaanker, M.; Malik, R.K.; McDonald, A.; Jain, M. Factors Constraining Timely Sowing of Wheat as an Adaptation to Climate Change in Eastern India. Weather Clim. Soc. 2020, 12, 515–528. [Google Scholar] [CrossRef]
- Sharma, R.K. Progress Report of AICRP on Wheat and Barley 2017–2018, Resource Management; ICAR-IIWBR Karnal: Karnal, India, 2018; p. 194. [Google Scholar]
- Anonymous. Progress Report of AICRP on Wheat and Barley 2018–2019, Resource Management; ICAR-IIWBR Karnal: Karnal, India, 2019. [Google Scholar]
- Ghuman, L.; Ram, H. Enhancing Wheat (Triticum aestivum L.) Grain Yield and Quality by Managing Lodging with Growth Regulators under Different Nutrition Levels. J. Plant Nutr. 2021, 44, 1916–1929. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.D.; Ortíz-Monasterios, I. Increasing Wheat Yields Sustainability through Agronomic Means; Natural Resources Group: West Sacramento, CA, USA, 1998. [Google Scholar]
- Peake, A.S.; Huth, N.I.; Carberry, P.S.; Raine, S.R.; Smith, R.J. Quantifying Potential Yield and Lodging-Related Yield Gaps for Irrigated Spring Wheat in Sub-Tropical Australia. Field Crops Res. 2014, 158, 1–14. [Google Scholar] [CrossRef]
- El-Debaby, A.; Ibrahim, K.; Saad, A.; El-Salhy, T. Wheat Lodging and Growth Characters as Affected by Some Agricultural Practices. Ann. Agric. Sci. Moshtohor. 1994, 32, 1325–1337. [Google Scholar]
- Berry, P.M.; Spink, J. Predicting Yield Losses Caused by Lodging in Wheat. Field Crops Res. 2012, 137, 19–26. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Yi, Y.; Ding, J.; Zhu, M.; Li, C.; Guo, W.; Feng, C.; Zhu, X. Effect of Nitrogen Levels and Nitrogen Ratios on Lodging Resistance and Yield Potential of Winter Wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0187543. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Lodging Yield Penalties as Affected by Breeding in Mediterranean Wheats. Field Crops Res. 2011, 122, 40–48. [Google Scholar] [CrossRef]
- Vera, C.L.; Duguid, S.D.; Fox, S.L.; Rashid, K.Y.; Dribnenki, J.C.P.; Clarke, F.R. Short Communication: Comparative Effect of Lodging on Seed Yield of Flax and Wheat. Can. J. Plant Sci. 2012, 92, 39–43. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Sayre, K.D.; Kaul, J.N.; Narang, R.S. Growth and Morphology of Spring Wheat (Triticum aestivum L.) Culms and Their Association with Lodging: Effects of Genotypes, N Levels and Ethephon. Field Crops Res. 2003, 84, 271–290. [Google Scholar] [CrossRef]
- Wei, F.; Li, J.; Wang, C.; Qu, H.; Shen, X. Effects of Nitrogenous Fertilizer Application Model on Culm Lodging Resistance in Winter Wheat. Acta Agron. Sin. 2008, 34, 1080–1085. [Google Scholar] [CrossRef]
- Berry, P.M.; Griffin, J.M.; Sylvester-Bradley, R.; Scott, R.K.; Spink, J.H.; Baker, C.J.; Clare, R.W. Controlling Plant Form through Husbandry to Minimise Lodging in Wheat. Field Crops Res. 2000, 67, 59–81. [Google Scholar] [CrossRef]
- Berry, P.M.; Spink, J.H. Understanding the Effect of a Triazole with Anti-Gibberellin Activity on the Growth and Yield of Oilseed Rape (Brassica napus). J. Agric. Sci. 2009, 147, 273–285. [Google Scholar] [CrossRef]
- Ramburan, S.; Greenfield, P.L. The Effects of Chlormequat Chloride and Ethephon on Agronomic and Quality Characteristics of South African Irrigated Wheat. S. Afr. J. Plant Soil 2007, 24, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Rajala, A.; Peltonen-Sainio, P. Plant Growth Regulator Effects on Spring Cereal Root and Shoot Growth. Agron. J. 2001, 93, 936–943. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Shearman, V.J.; Sylvester-Bradley, R.; Scott, R.K.; Foulkes, M.J. Physiological Processes Associated with Wheat Yield Progress in the UK. Crop Sci. 2005, 45, 175–185. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Yadav, M.; Sexena, R.; Gupta, K.; Garg, N.; Yadav, H. Impact of Nutrient Management Practices and Plant Growth Regulators on Growth, Productivity and Profitability of Wheat (Triticum aestivum). Indian J. Agric. Sci. 2019, 89, 604–609. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Mamrutha, H.M.; Singh, R.; Sharma, D.; Venkatesh, K.; Pandey, G.C.; Kumar, R.; Tiwari, R.; Sharma, I. Physiological and Molecular Basis of Abiotic Stress Tolerance in Wheat. In Genetic Enhancement of Crops for Tolerance to Abiotic Stress: Mechanisms and Approaches, Volume I; Rajpal, V.R., Sehgal, D., Kumar, A., Raina, S.N., Eds.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, Switzerland, 2019; Volume 20, pp. 99–124. ISBN 978-3-319-91955-3. [Google Scholar]
- IBM. IBM SPSS Statistics for Windows; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Ahmed, M.; Ahmad, S.; Ashraf, M.; Gill, M.A. Partial Budgeting of Different Sowing Technologies of Wheat. Sarhad J. Agric. 2007, 23, 1291. [Google Scholar]
- David, R.C.; Hir, S.P.; Ramesh, K.G.; Subash, C.; Ra, G.; Anuj, K.; Ramesh, K.S.; Ramesh, C.; Sendhil, R. Enhancing Farm Profitability by Growing Wheat for Chapatti Quality Markets in Haryana, India. Afr. J. Agric. Res. 2013, 8, 6265–6274. [Google Scholar]




| SN | Genotype | Gurdaspur | Ludhiana | Ladhowal | Karnal | Delhi | Pantnagar | Zonal Mean | % Gain over Best Check | Zonal Mean under RDF |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HD3317 | 63.4 | 82.1 | 63.3 | 84.5 | 73.0 | 74.0 | 73.7 | −2.51 | 65.8 |
| 2 | WH1254 | 60.1 | 75.4 | 69.9 | 80.4 | 66.0 | 72.4 | 70.7 | −6.48 | 72.2 |
| 3 | DBW301 | 66.7 | 79.1 | 68.4 | 78.0 | 71.4 | 66.6 | 71.7 | −5.16 | 62.3 |
| 4 | WH1270 | 78.3 | 84.6 | 67.5 | 91.5 | 77.2 | 71.8 | 78.5 | 3.84 | 71.4 |
| 5 | PBW824 | 66.5 | 87.2 | 68.0 | 98.8 | 68.4 | 71.1 | 76.7 | 1.46 | 69.7 |
| 6 | UP3043 | 67.2 | 74.8 | 70.1 | 92.9 | 77.0 | 77.7 | 76.6 | 1.32 | 71.3 |
| 7 | DBW187 | 75.6 | 77.4 | 74.6 | 96.6 | 70.9 | 76.5 | 78.6 | 3.97 | 73.0 |
| 8 | DBW303 | 73.0 | 91.9 | 70.9 | 97.4 | 69.7 | 79.2 | 80.4 | 6.35 | 71.3 |
| 9 | DBW304 | 66.3 | 77.4 | 49.5 | 87.8 | 62.2 | 71.0 | 69.0 | −8.73 | 64.3 |
| 10 | UP3042 | 68.3 | 86.7 | 64.7 | 96.4 | 73.2 | 72.3 | 76.9 | 1.72 | 69.1 |
| 11 | DBW302 | 60.1 | 79.2 | 69.4 | 89.2 | 70.4 | 79.3 | 74.6 | −1.32 | 66.7 |
| 12 | PBW825 | 69.1 | 82.4 | 67.7 | 86.2 | 75.1 | 68.4 | 74.8 | −1.1 | 65.6 |
| 13 | HD3347 | 62.6 | 81.9 | 63.5 | 85.2 | 67.0 | 67.3 | 71.2 | −5.82 | 67.6 |
| 14 | HD2967(C) | 38.1 | 78.4 | 67.8 | 81.6 | 59.5 | 72.0 | 65.9 | −12.83 | 64.7 |
| 15 | HD3086(C) | 69.9 | 80.5 | 70.4 | 88.3 | 70.3 | 73.9 | 75.6 | − | 70.5 |
| Grand Mean | 65.7 | 81.3 | 67.2 | 89.0 | 70.0 | 72.9 | 74.3 | − | 68.4 | |
| AVT-IR-TS | − | − | − | − | − | − | 62.2 | − | ||
| SE | 1.94 | 3.23 | 2.09 | 1.91 | 3.11 | 0.47 | 0.95 | − | − | |
| CD | 4.6 | 7.7 | 5.0 | 4.6 | 7.4 | 1.1 | 2.2 | − | − | |
| CV | 5.9 | 8.0 | 6.2 | 4.3 | 8.9 | 1.3 | − | − | ||
| Genotypes | DTH (Days) | DTM (Days) | GFD (Days) | Fv/Fm | NBI | FLV | CCI | CT (°C) | NDVI |
|---|---|---|---|---|---|---|---|---|---|
| HD3717 | 115 ± 2.6 b | 156 ± 2.7 a | 42 ± 0.28 b | 0.787 ± 0.012 abc | 33.08 ± 4.01 b | 1.258 ± 0.10 ab | 39.6 ± 1.8 a | 26.6 ± 1.3 a | 0.905 ± 0.003 abc |
| WH1254 | 111± 1.9 b | 152 ± 1.7 c | 42 ± 0.28 b | 0.767 ± 0.014 bc | 30.05 ± 4.64 ab | 1.218 ± 0.07 ab | 35.4 ± 6.6 a | 26.3 ± 1.3 a | 0.895 ± 0.009 abc |
| DBW301 | 119 ± 5.7 a | 148± 1.7 d | 29 ± 3.94 f | 0.810± 0.004 ab | 25.27 ± 0.16 ab | 1.355 ± 0.05 ab | 33.9 ± 2.0 a | 27.9 ± 1.2 a | 0.910 ± 0.004 a |
| WH1270 | 108 ± 2.6 fg | 147 ± 4.0 gh | 39 ± 1.43 d | 0.812 ± 0.006 ab | 41.20 ± 1.58 a | 1.013 ± 0.05 b | 41 ± 1.0 a | 27.2 ± 1.7 a | 0.887 ± 0.006 cdef |
| HD2967 (C) | 115 ± 3.1 be | 154 ± 1.7 b | 40 ± 1.65 cd | 0.777 ± 0.011 bc | 35.28 ± 7.34 ab | 1.390 ± 0.15 ab | 32.0 ± 5.2 a | 27.5 ± 1.7 a | 0.900 ± 0.004 abcd |
| PBW824 | 109 ± 4.7 h | 149 ± 0.2 de | 40± 4.42 a | 0.786 ± 0.010 abc | 27.58 ± 2.39 ab | 1.265 ± 0.06 ab | 34.7 ± 1.9 a | 27.6 ± 1.0 a | 0.890 ± 0.004 bcde |
| UP3043 | 105± 0.3 gh | 145 ± 1.0 f | 41 ± 0.95 b | 0.789 ± 0.005 abc | 31.65 ± 4.51 ab | 1.585 ± 0.12 a | 36.7 ± 3.3 a | 27.1 ± 1.0 a | 0.883 ± 0.006 ef |
| DBW187 | 108 ± 0.8 ef | 148 ± 0.2 e | 41 ± 0.62 b | 0.782 ± 0.011 abc | 36.62 ± 2.45 ab | 1.058 ± 0.05 b | 38.0 ± 1.2 a | 27.8 ± 1.0 a | 0.882 ± 0.005 def |
| HD3086 (C) | 105 ± 4.4 j | 145 ± 3.3 h | 40 ± 1.10 b | 0.759 ± 0.012 c | 29.17 ± 1.59 ab | 1.160 ± 0.09 ab | 32.0 ± 0.9 a | 27.9 ± 1.7 a | 0.893 ± 0.007 bcdef |
| DBW303 | 103 ± 1.0 i | 143 ± 0.5 g | 41 ± 1.25 b | 0.779 ± 0.005 abc | 32.85 ± 2.54 ab | 1.185 ± 0.04 ab | 38 ± 2.1 a | 27.1 ± 2.3 a | 0.885 ± 0.003 def |
| DBW304 | 109 ± 4.4 h | 148 ± 3.7 g | 39 ± 0.70 bc | 0.795 ± 0.009 abc | 30.52 ± 1.27 ab | 1.183 ± 0.02 b | 35.6 ± 2.4 a | 28.8 ± 2.3 a | 0.890 ± 0.004 bcdef |
| UP3042 | 109 ± 0.6 d | 148 ± 0.4 e | 39 ± 0.94 cd | 0.771 ± 0.014 bc | 28.37 ± 4.73 ab | 1.228 ± 0.09 ab | 32.6 ± 3.9 a | 26.3 ± 0.3 a | 0.908 ± 0.002 ab |
| DBW302 | 121 ± 3.0 a | 157 ± 2.2 a | 36± 0.85 e | 0.819 ± 0.005 a | 27.81 ± 0.41 ab | 1.315 ± 0.03 ab | 36.5 ± 0.7 a | 26.7 ± 0.5 a | 0.908 ± 0.006 ab |
| PBW825 | 112 ± 0.5 b | 151 ± 1.2 d | 39 ± 1.03 cd | 0.768 ± 0.003 bc | 36.75 ± 2.78 ab | 1.098 ± 0.10 b | 39.5 ± 2.1 a | 26.6 ± 1.5 a | 0.883 ± 0.009 f |
| HD3347 | 106 ± 1.9 | 147 ± 1.5 e | 41 ± 0.47 b | 0.794 ± 0.002 abc | 29.28 ± 0.63 ab | 1.213 ± 0.03 ab | 35.2 ± 1.8 a | 27.8 ± 0.7 a | 0.900 ± 0.007 abc |
| CD | 8.922 | 6.320 | 5.265 | 0.025 | N/A | 0.247 | N/A | N/A | 0.016 |
| SE | 3.115 | 2.207 | 1.838 | 0.009 | 3.102 | 0.086 | 3.02 | 1.38 | 0.006 |
| CV | 5.650 | 2.950 | 9.390 | 2.240 | 19.57 | 13.950 | 4.26 | 1.95 | 1.25 |
| Genotypes | BM (kg) | HI | GNS | GWS (g) | TGW (g) | PH (cm) | SPKL (cm) | TN |
|---|---|---|---|---|---|---|---|---|
| HD3717 | 32.7 ± 0.6 ab | 38.5 ± 1.21 cd | 51 ± 0.5 e | 2.5 ± 0.0 d | 48.9 ± 0.4 def | 104.0 ± 2.273 ab | 10.2 ± 0.49 cd | 113 ± 2.9 abc |
| WH1254 | 31.2 ± 1.5 b | 38.5 ± 0.46 bc | 42 ± 0.3 i | 2.4 ± 0 i | 40 ± 0.5 i | 100.2 ± 1.601 cdef | 11.8 ± 0.71 abcd | 113 ± 9.1 abc |
| DBW301 | 32.6 ± 1.0 ab | 35.9 ± 2.19 d | 53 ± 0.2 cd | 2.0 ± 0.0 h | 40.3 ± 0.3 i | 98.5 ± 2.327 f | 13.57 ± 0.17 a | 105 ± 11.8 abc |
| WH1270 | 33.9 ± 1.7 ab | 37.8 ± 1.67 bc | 48 ± 0.3 f | 2.4 ± 0.0 de | 50.1 ± 0.4 cd | 95.7 ± 1.315 ef | 11.40 ± 0.44 abcd | 114 ± 8.7 abc |
| HD2967 (C) | 32.5 ± 0.7 ab | 37.9 ± 1.08 cd | 45 ± 0.6 h | 2.0 ± 0.0 h | 45.1 ± 0.4 h | 104.2 ± 2.056 ab | 11.22 ± 0.66 bcd | 102 ± 13.4 abc |
| PBW824 | 32.8 ± 2.1 a | 41.9 ± 1.20 abc | 45 ± 0.3 gh | 2.1 ± 0.0 gh | 45.5 ± 0.2 h | 100.8 ± 1.887 bce | 11.17 ± 0.08 bcd | 101 ± 4.9 abc |
| UP3043 | 34 ± 1.1 ab | 39.3 ± 1.68 abc | 53 ± 0.5 c | 2.8 ± 0.03 bc | 53.0 ± 0.5 b | 108.0 ± 2.121 ab | 10.80 ± 0.28 bcd | 89 ± 7.7 c |
| DBW187 | 34.2 ± 1.7 ab | 41.5 ± 1.30 ab | 47 ± 0.5 fg | 2.9 ± 0 b | 62 ± 0.5 a | 104.0 ± 2.160 bcd | 11.05 ± 0.73 bcd | 126 ± 18.3 ab |
| HD3086 (C) | 30.7 ± 1.2 b | 40.7 ± 1.89 ab | 42 ± 0.6 j | 2.4 ± 0 j | 46.8 ± 0.2 g | 98.8 ± 1.109 cdef | 10.82 ± 0.51 bcd | 133 ± 3.1 a |
| DBW303 | 32.5 ± 0.9 ab | 42.8 ± 0.70 a | 65 ± 0.9 a | 3.2 ± 0.0 a | 48.2 ± 0.4 ef | 98.5 ± 1.041 def | 10.95 ± 0.67 bcd | 103 ± 6.2 abc |
| DBW304 | 32.3 ± 1.3 ab | 39.5 ± 0.73 abc | 51 ± 0.4 e | 2.5 ± 0.0 d | 49.2 ± 0.6 cde | 101.0 ± 2.041 cdef | 12.57 ± 0.32 abc | 109 ± 11.8 abc |
| UP3042 | 33.5 ± 1.2 ab | 39.5 ± 1.34 abc | 47 ± 0.2 fg | 2.3 ± 0.03 ef | 50.4 ± 0.3 c | 105.7 ± 2.097 abc | 9.72 ± 0.37 d | 96 ± 2.1 abc |
| DBW302 | 32.9 ± 1.3 ab | 38.9 ± 1.79 cd | 58 ± 0.2 b | 2.8 ± 0.0 b | 48.5 ± 0.2 ef | 109.5 ± 1.936 ab | 11.00 ± 0.28 bcd | 129 ± 3.7 ab |
| PBW825 | 30.6 ± 0.5 b | 39.4 ± 1.44 abc | 52 ± 0.6 de | 2.7 ± 0.0 c | 50.2 ± 0.4 cd | 101.7 ± 2.175 bcd | 11.55 ± 0.60 abcd | 103 ± 12.8 abc |
| HD3347 | 30.7 ± 0.7 b | 40.3 ± 0.75 abc | 44 ± 0.3 h | 2.2 ± 0.0 fg | 47.8 ± 0.3 fg | 98.7 ± 1.031 def | 12.67 ± 0.40 ab | 104 ± 11.8 bc |
| CD | N/A | N/A | 1.437 | 0.146 | 1.154 | 5.315 | 1.399 | N/A |
| SE | 1.16 | 1.369 | 0.49 | 0.05 | 0.396 | 1.856 | 0.488 | 9.36 |
| CV | 7.17 | 6.92 | 0.70 | 0.071 | 0.56 | 3.64 | 8.58 | 17.09 |
| Traits | r | p Values |
|---|---|---|
| PH | 0.24 | 0.393 |
| TN | −0.33 | 0.23 |
| DTH | −0.63 | 0.013 * |
| DTM | −0.45 | 0.096 |
| GFD | 0.51 | 0.04 * |
| NDVI | 0.27 | 0.047 * |
| BM | 0.73 | 0.002 ** |
| HI | 0.75 | 0.001 ** |
| FV/Fm | −0.05 | 0.862 |
| NBI | 0.22 | 0.435 |
| CCI | 0.32 | 0.251 |
| FLAV | −0.38 | 0.164 |
| CT | 0.27 | 0.326 |
| SPKL | −0.20 | 0.473 |
| GNS | 0.23 | 0.405 |
| GWS | 0.48 | 0.068 |
| TGW | 0.62 | 0.014 * |
| Additional Cost (Annualized) | USD | Additional Returns (Annualized) | USD |
|---|---|---|---|
| 1. 150% RFD (Nutrients Cost) | 31.90 | 1. Grain (+12.1 Q/ha) | 316.29 |
| 2. Lihocin: 2 L/ha | 25.57 | 2. Straw (+15.69 Q/ha) | 89.16 |
| 3. Folicur 430: 1 L/ha | 31.25 | -- | -- |
| 4. FYM Cost: 15 t/ha | 68.19 | -- | -- |
| 5. Labour Cost for all inputs | 46.60 | -- | -- |
| Reduced Returns (annualized) | USD | Reduced Cost (annualized) | USD |
| Nil | -- | Nil | -- |
| Total Negative Effects | 203.51 | Total Positive Effects | 405.46 |
| Net Gain | 201.95 | ||
| SN | Genotype | Pedigree |
|---|---|---|
| 1. | HD3317 | 31stESWYT-117//DW1272/HP1731 |
| 2. | WH1254 | PRL/2*PASTOR//PBW343*2/KUKUNA/WHEAR//INQALAB91*2/TUKURU//SOKOLL*2/4/CHEN/AEGILOPS SQUARROSA(TAUS.) |
| 3. | DBW301 | SR39/DPW621-50 |
| 4. | WH1270 | SHA7//PRL/VEE#6/3/FASAN/4/HAAS8446/2*FASAN/5/CBRD/KAUZ/6/MILAN/AMSEL/7/FRET2*2/KUKUNA/8/2*WHEAR/SOKOLL |
| 5. | PBW824 | WAXWING//INQALAB91*2/KUKUNA/3/WBLL1*2/TUKURU/8/2*NG8201/KAUZ/4/SHA7//PRL/VEE#6/3/FASAN/5/MILAN/KAUZ/6/ACHYUTA/7/PBW343*2/KUKUNA |
| 6. | UP3043 | CHIBIA//PRLII/CM65531/3/SKAUZ/BAV92*2/4/HUW234+LR34/PRINIA//PBW343*2/KUKUNA/3/ROLF07 |
| 7. | DBW187 | NAC/TH.AC//3*PVN/3/MIRLO/BUC/4/2*PASTOR/5/KACHU/6/KACHU |
| 8. | DBW303 | WBLL1*2/BRAMBLING/4/BABAX/LR42//BABAX*2/3/SHAMA*2/5/PBW343*2/KUKUNA*2//FRTL/PIFED |
| 9. | DBW304 | ADI/3/KINGBIRD#1//INQALAB91*2/TUKURU/4/NADI |
| 10. | UP3042 | CAL/NH//H567.71/3/SERI/4/CAL/NH//H567.71/5/2*KAUZ/6/WH576/7/WH542/8/WAXWING/9/ATTILA*2/PBW65/6/PVN//CAR422/ANA/5/BOW/CROW//BUC/PVN/3/YR/4/TRAP#1/7/ATTILA/2*PASTOR/10/UP2338*2/KKTS*2//YA/NAC |
| 11. | DBW302 | DBW112/HD3108 |
| 12. | PBW825 | SAUAL/MUTUS*2//PICAFLOR #1 |
| 13. | HD3347 | HD3086/HD2997 |
| 14. | HD2967 | ALD/CUC//URES/HD2160M/HD2278 |
| 15. | HD3086 | DBW14/HD2733//HUW468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.; Mamrutha, H.M.; Mishra, C.N.; Krishnappa, G.; Sendhil, R.; Parkash, O.; Joshi, A.K.; Chatrath, R.; Tyagi, B.S.; Singh, G.; et al. Harnessing High Yield Potential in Wheat (Triticum aestivum L.) under Climate Change Scenario. Plants 2023, 12, 1271. https://doi.org/10.3390/plants12061271
Khan H, Mamrutha HM, Mishra CN, Krishnappa G, Sendhil R, Parkash O, Joshi AK, Chatrath R, Tyagi BS, Singh G, et al. Harnessing High Yield Potential in Wheat (Triticum aestivum L.) under Climate Change Scenario. Plants. 2023; 12(6):1271. https://doi.org/10.3390/plants12061271
Chicago/Turabian StyleKhan, Hanif, Harohalli Masthigowda Mamrutha, Chandra Nath Mishra, Gopalareddy Krishnappa, Ramadas Sendhil, Om Parkash, Arun Kumar Joshi, Ravish Chatrath, Bhudeva Singh Tyagi, Gyanendra Singh, and et al. 2023. "Harnessing High Yield Potential in Wheat (Triticum aestivum L.) under Climate Change Scenario" Plants 12, no. 6: 1271. https://doi.org/10.3390/plants12061271
APA StyleKhan, H., Mamrutha, H. M., Mishra, C. N., Krishnappa, G., Sendhil, R., Parkash, O., Joshi, A. K., Chatrath, R., Tyagi, B. S., Singh, G., & Singh, G. P. (2023). Harnessing High Yield Potential in Wheat (Triticum aestivum L.) under Climate Change Scenario. Plants, 12(6), 1271. https://doi.org/10.3390/plants12061271