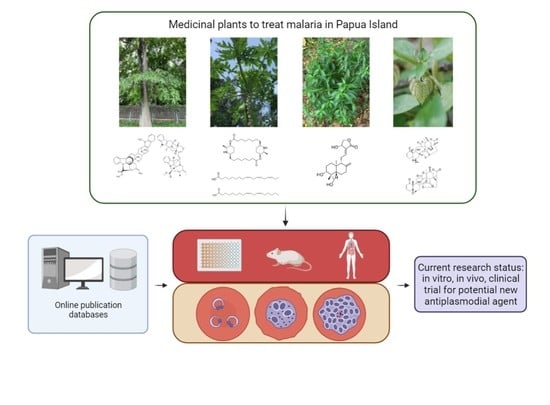
Potential Plant-Based New Antiplasmodial Agent Used in Papua Island, Indonesia
Abstract
:1. Introduction
2. Methods
3. Alstonia scholaris (L.) R. Br.
3.1. Ethnopharmacology
3.2. Phytochemistry
3.3. Antiplasmodial Activity
| Plant | Plant’s Part Used | Solvent | Extraction Method | Assay | Dose/ED50/IC50 | Active Compound | Reference |
|---|---|---|---|---|---|---|---|
| Alstonia scholaris | Stem Bark | Ethanol 95% | Maceration (triplicate) | In vitro (P. falciparum chloroquine-sensitive 3D7 strain) | IC50: 15.46 µg/mL (Moderate) | - | [43] |
| Stem Bark | Ethanol 70% | Maceration (triplicate) | In vitro (P. falciparum chloroquine-sensitive 3D7 strain) | IC50: 0.165 µg/mL (Strong) | - | [44] | |
| In vivo (P. berghei, NK 65 strain in mice) | ED50: 121.94 mg/kg BW (Good) | - | |||||
| Leaves | Methanol | Percolation | In vitro (P. falciparum chloroquine-resistant K1) | IC50: 210.8 µg/mL | - | [46] | |
| Stem Bark | IC50: 181.4 µg/mL | - | |||||
| Root Bark | IC50: 370.2 µg/mL | - | |||||
| Isolates * | - | - | IC50: 0.27 µg/mL (1) IC50: 0.36 µg/mL (2) | (1) Villalstonine (2) Macrocarpamine | |||
| Carica papaya | Leaves | Ethanol 70% | Maceration (triplicate) | In vitro (P. falciparum chloroquine-sensitive 3D7 strain) | IC50: 0.177 µg/mL (Strong) | - | [44] |
| In vivo (P. berghei, NK65 strain in mice) | ED50: 173.20 mg/kg BW (Good) | - | |||||
| Leaves | Ethanol | Percolation | In vitro (P. falciparum) | IC50: 46.23 µg/mL | - | [53] | |
| Stem | IC50: 65.13 µg/mL | - | |||||
| Leaves | Methanol 50% | Not mentioned | In vivo (P. berghei, NK65 strain in mice) | Dose: 100 mg/kg BW (>50% chemosuppresion) | - | [54] | |
| Leaves | Sequential solvent: petroleum ether followed by dichlorometane, ethyl acetate, methanol, and water | Maceration (4-5 times, shaken continuously) | In vitro (P. falciparum chloroquine-sensitive D10) | IC50: 2.6 µg/mL (ethyl acetate) IC50: 10.8 µg/mL (methanol) IC50: 12.8 µg/mL (dichloromethane) IC50: 16.4 µg/mL (petroleum ether) IC50 > 50 µg/mL (water) | - | [55] | |
| Isolates | - | - | In vitro (P. falciparum chloroquine-sensitive D10) | IC50: 3.58 µg/mL (1) IC50: 6.88 µg/mL (2) | (1) Linolenic Acid (2) Linoleic Acid | ||
| In vitro (P. falciparum chloroquine-resistant Dd2 strain) | IC50: 4.40 µg/mL (1) IC50: 6.80 µg/mL (2) | ||||||
| Isolate | - | - | In vitro (P. falciparum) | IC50: 0.2 µM | Carpaine | [56] | |
| In vivo (P. berghei ANKA strain) | Dose: not specified, resulting only 11.9% reduction in parasitaemia | Carpaine | |||||
| Isolate | - | - | In vitro (P. falciparum chloroquine-sensitive 3D7) | IC50: 2.01 µg/mL | Carpaine | [57] | |
| In vitro (P. falciparum chloroquine-resistant Dd2 strain) | IC50: 2.19 µg/mL | ||||||
| Fruit rind/peel | Chloroform | Soxhlet | In vivo (P. berghei chloroquine-sensitive ANKA strain) | Dose: 400 mg/kg BW (61.78% Chemosuppresion, moderate) | - | [58] | |
| Methanol | Dose: 400 mg/kg BW (37.65% Chemosuppresion, Mild) | - | |||||
| Petroleum ether | Dose: 400 mg/kg BW (18.39% Chemosuppresion, Weak) | - | |||||
| Root | Chloroform | Soxhlet | In vivo (P. berghei chloroquine-sensitive ANKA strain) | Dose: 400 mg/kg BW (43.77% Chemosuppresion, Mild) | - | ||
| Methanol | Dose: 400 mg/kg BW (48.11% Chemosuppresion, Mild) | - | |||||
| Water | Dose: 400 mg/kg BW (25.63% Chemosuppresion, Mild) | - | |||||
| Leaves | Water | Maceration | In vivo (P. berghei, NK65 strain in mice) | Dose: 350 mg/kg BW | - | [59] | |
| Leaves | Ethanol | Soxhlet | In vitro (P. falciparum chloroquine-sensitive) | IC50: 25–150 µg/mL | - | [60] | |
| In vitro (P. falciparum chloroquine-resistant) | IC50: 25–150 µg/mL | - | |||||
| Andrographis paniculata | Herb (aerial parts) | Purified ethyl acetate fraction from Ethanol 96% extract | Maceration followed by liquid-liquid fractionation and further purification | In vivo (P. berghei chloroquine-sensitive ANKA strain) | Dose: 15 mg purified fraction per 300 mg tablet by wet granulation (78.16% inhibition) and 60 mg purified fraction per 150 mg tablet by solid dispersion method (80.35% inhibition) | - | [61] |
| Herb (aerial parts) | Ethyl acetate fraction from ethanol 96% extract | Maceration followed by liquid-liquid fractionation | Clinical trial Phase 1 | Equivalent to 35 mg andrographolide per 380 mg granule. 4 tablets twice daily for 4 days, classified as non-toxic & safe | - | [62] | |
| Herb (aerial parts) | Ethanol 50% | Percolation | In vitro (P. falciparum of Papua strain (2300) | Optimal dose at 200 µg/mL (10% parasitemia) | - | [63] | |
| Not mentioned | Ethanol 50% | Percolation | Clinical trial Phase 2 | Dose: 250 mg extract per 460 mg capsule → 94.2% (65 of 69) patients have negative parasitaemia on day 7 | - | [64] | |
| Herb (aerial parts) | Methanol | Stirred at 4 °C overnight | In vitro (P. falciparum chloroquine-sensitive MRC-pf-20 and chloroquine-resistant MRC-pf-303) | IC50: 7.2 µg/mL for both strain | - | [65] | |
| In vivo (P. berghei ANKA strain) | Dose: 7 mg/Kg BW, 39% parasitaemia at 12th day, control mice all died | ||||||
| Isolate | - | - | In vitro (P. falciparum chloroquine-resistant MRC-pf-303) | IC50: 9.1 µM | Andrographolide | [66] | |
| In vivo (P. bergheii ANKA strain) | Dose: 15 mg/Kg BW, 46% parasitaemia at day 13–15, control mice all died | ||||||
| Isolate | - | - | In vitro (P. falciparum 3D7) | IC50: 13.70 µM | Andrographolide | [67] | |
| In vivo (P. berghei chloroquine-sensitive NK65) | Dose: 5 mg/kg BW, 60.17% chemosuppresion | ||||||
| Whole plant | n-Hexane | Soxhlet | In vitro (P. falciparum FCR-3 strain) | - | - | [68] | |
| Chloroform | 100% inhibition at 0.05 mg/mL after 24 h | ||||||
| Methanol | 100% inhibition at 25 mg/mL after 48 h | ||||||
| Whole plant | n-Hexane | Soxhlet | In vivo (P. berghei ANKA) | - | - | ||
| Chloroform | - | ||||||
| Methanol | 5 mg/Kg BW → delayed parasetaemia and all died at day 7 where control died at day 5 | ||||||
| Physalis minima | Isolate * | - | - | In vitro (P. falciparum W2 clone) | IC50: 2.8 µg/mL | Physalin B | [69] |
| IC50: 55 µg/mL | Physalin D | ||||||
| IC50: 2.2 µg/mL | Physalin F | ||||||
| IC50: 6.7 µg/mL | Physalin G | ||||||
| In vivo (P. berghei strain NK65) | Dose: 100 mg/kg → no decrease in parasitemia | Physalin F | |||||
| Dose: 100 mg/kg → 65% decrease in parasitemia | Physalin D |
4. Carica papaya L.
4.1. Ethnopharmacology
4.2. Phytochemistry
4.3. Antiplasmodial Activity
5. Andrographis paniculata (Burm. f.)
5.1. Ethnopharmacology
5.2. Phytochemistry
5.3. Antiplasmodial Activity
6. Physalis minima L.
6.1. Ethnopharmacology
6.2. Phytochemistry
6.3. Antiplasmodial Activity
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. World Malaria Report 2020—20 Years of Global Progress & Challenges; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kementerian Kesehatan Republik Indonesia. Profil Kesehatan Indonesia Tahun 2020; Kementerian Kesehatan Republik Indonesia: Jakarta, Indonesia, 2021. [Google Scholar]
- Naß, J.; Efferth, T. Development of Artemisinin Resistance in Malaria Therapy. Pharmacol. Res. 2019, 146, 104275. [Google Scholar] [CrossRef]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431–454. [Google Scholar] [CrossRef]
- Buyon, L.E.; Elsworth, B.; Duraisingh, M.T. The Molecular Basis of Antimalarial Drug Resistance in Plasmodium vivax. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 23–37. [Google Scholar] [CrossRef]
- Elyazar, I.R.F.; Hay, S.I.; Baird, J.K. Malaria Distribution, Prevalence, Drug Resistance and Control in Indonesia Europe PMC Funders Group. Adv. Parasitol. 2011, 74, 41–175. [Google Scholar] [CrossRef]
- Fagbemi, K.A.; Adebusuyi, S.A.; Nderu, D.; Adedokun, S.A.; Pallerla, S.R.; Amoo, A.O.J.; Thomas, B.N.; Velavan, T.P.; Ojurongbe, O. Analysis of Sulphadoxine–Pyrimethamine Resistance-Associated Mutations in Plasmodium falciparum Isolates Obtained from Asymptomatic Pregnant Women in Ogun State, Southwest Nigeria. Infect. Genet. Evol. 2020, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Elfahmi; Woerdenbag, H.J.; Kayser, O. Jamu: Indonesian Traditional Herbal Medicine towards Rational Phytopharmacological Use. J. Herb. Med. 2014, 4, 51–73. [Google Scholar] [CrossRef]
- von Rintelen, K.; Arida, E.; Häuser, C. A Review of Biodiversity-Related Issues and Challenges in Megadiverse Indonesia and Other Southeast Asian Countries. Res. Ideas Outcomes 2017, 3, e20860. [Google Scholar] [CrossRef]
- Elyazar, I.R.F.; Gething, P.W.; Patil, A.P.; Rogayah, H.; Kusriastuti, R.; Wismarini, D.M.; Tarmizi, S.N.; Baird, K.J.; Hay, S.I. Plasmodium falciparum Malaria Endemicity in Indonesia in 2010. PLoS ONE 2011, 6, e0021315. [Google Scholar] [CrossRef]
- Budiarti, M.; Maruzy, A.; Mujahid, R.; Sari, A.N.; Jokopriyambodo, W.; Widayat, T.; Wahyono, S. The Use of Antimalarial Plants as Traditional Treatment in Papua Island, Indonesia. Heliyon 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Khyade, M.S.; Kasote, D.M.; Vaikos, N.P. Alstonia Scholaris (L.) R. Br. and Alstonia macrophylla Wall. Ex G. Don: A Comparative Review on Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2014, 153, 1–18. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yang, Z.F.; Wu, B.F.; Shang, J.H.; Liu, Y.P.; Wang, X.H.; Luo, X.D. Indole Alkaloids from Leaves of Alstonia scholaris (L.) R. Br. Protect against Emphysema in Mice. J. Ethnopharmacol. 2020, 259, 112949. [Google Scholar] [CrossRef]
- iNaturalist. Alstonia scholaris (L.) R.Br. Observed in Singapore by Amodnargund. Available online: https://www.inaturalist.org/observations/107359940 (accessed on 16 January 2023).
- iNaturalist. Alstonia scholaris (L.) R.Br. Observed in Chinese Taipei by Hong. Available online: https://www.inaturalist.org/observations/114569913 (accessed on 16 January 2023).
- iNaturalist. Alstonia scholaris (L.) R.Br. Observed in Australia by Upollo. Available online: https://www.inaturalist.org/observations/124224199 (accessed on 16 January 2023).
- Pratap, B.; Chakraborthy, G.; Mogha, N. Complete Aspects of Alstonia scholaris. Int. J. Pharm. Tech. Res. 2013, 5, 17–26. [Google Scholar]
- Dey, A. Alstonia scholaris R.Br. (Apocynaceae): Phytochemistry and Pharmacology: A Concise Review Abhijit Dey. J. Appl. Pharm. Sci. 2011, 6, 51–57. [Google Scholar]
- Arulmozhi, S.; Mitra Mazumder, P.; Ashok, P.; Sathiya Narayanan, L. Pharmacological Activities of Alstonia scholaris Linn. (Apocynaceae)-A Review. Pharmacogn. Rev. 2007, 1, 163–170. [Google Scholar]
- Shang, J.H.; Cai, X.H.; Feng, T.; Zhao, Y.L.; Wang, J.K.; Zhang, L.Y.; Yan, M.; Luo, X.D. Pharmacological Evaluation of Alstonia scholaris: Anti-Inflammatory and Analgesic Effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef]
- Dinas Kesehatan Provinsi Papua. Tumbuhan Obat Tradisional Papua; Dinas Kesehatan Provinsi Papua: Jayapura, Indonesia, 2016; ISBN 978-602-60288-9-1. [Google Scholar]
- Shrinath Baliga, M. Alstonia scholaris Linn R Br in the Treatment and Prevention of Cancer: Past, Present, and Future. Integr. Cancer Ther. 2010, 9, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.H.; Du, Z.Z.; Luo, X.D. Unique Monoterpenoid Indole Alkaloids from Alstonia scholaris. Org. Lett. 2007, 9, 1817–1820. [Google Scholar] [CrossRef]
- Cai, X.H.; Liu, Y.-P.; Feng, T.; Luo, X.-D. Picrinine-Type Alkaloids from the Leaves of Alstonia scholaris. Chin. J. Nat. Med. 2008, 6, 20–22. [Google Scholar] [CrossRef]
- Cai, X.H.; Tan, Q.G.; Liu, Y.P.; Feng, T.; Du, Z.Z.; Li, W.Q.; Luo, X.D. A Cage-Monoterpene Indole Alkaloid from Alstonia scholaris. Org. Lett. 2008, 10, 577–580. [Google Scholar] [CrossRef]
- Cai, X.H.; Shang, J.H.; Feng, T.; Luoa, X.D. Novel Alkaloids from Alstonia scholaris. Z. Naturforsch. 2010, 65, 1164–1168. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yang, J.; Yang, X.W.; Khan, A.; Liu, L.; Wang, B.; Zhao, Y.L.; Liu, Y.P.; Ding, Z.T.; Luo, X.D. Alstorisine A, a nor-Monoterpenoid Indole Alkaloid from Cecidogenous Leaves of Alstonia scholaris. Tetrahedron Lett. 2016, 57, 1754–1757. [Google Scholar] [CrossRef]
- Feng, T.; Cai, X.H.; Zhao, P.J.; Du, Z.Z.; Li, W.Q.; Luo, X.D. Monoterpenoid Indole Alkaloids from the Bark of Alstonia scholaris. Planta Med. 2009, 75, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, Y.Y.; Qin, X.J.; Wang, B.; Jin, Q.; Liu, Y.P.; Luo, X.D. Antibacterial Monoterpenoid Indole Alkaloids from Alstonia scholaris Cultivated in Temperate Zone. Fitoterapia 2015, 105, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qin, X.J.; Liu, Y.P.; Wu, T.; Luo, X.D.; Xia, C. Alstoscholarisines H-J, Indole Alkaloids from Alstonia scholaris: Structural Evaluation and Bioinspired Synthesis of Alstoscholarisine H. Org. Lett. 2016, 18, 654–657. [Google Scholar] [CrossRef]
- Qin, X.J.; Zhao, Y.L.; Lunga, P.K.; Yang, X.W.; Song, C.W.; Cheng, G.G.; Liu, L.; Chen, Y.Y.; Liu, Y.P.; Luo, X.D. Indole Alkaloids with Antibacterial Activity from Aqueous Fraction of Alstonia scholaris. Tetrahedron 2015, 71, 4372–4378. [Google Scholar] [CrossRef]
- Qin, X.J.; Zhao, Y.L.; Song, C.W.; Wang, B.; Chen, Y.Y.; Liu, L.; Li, Q.; Li, D.; Liu, Y.P.; Luo, X.D. Monoterpenoid Indole Alkaloids from Inadequately Dried Leaves of Alstonia scholaris. Nat. Prod. Bioprospect. 2015, 5, 185–193. [Google Scholar] [CrossRef]
- Yang, X.W.; Qin, X.J.; Zhao, Y.L.; Lunga, P.K.; Li, X.N.; Jiang, S.Z.; Cheng, G.G.; Liu, Y.P.; Luo, X.D. Alstolactines A–C, Novel Monoterpenoid Indole Alkaloids from Alstonia scholaris. Tetrahedron Lett. 2014, 55, 4593–4596. [Google Scholar] [CrossRef]
- Yang, X.W.; Yang, C.P.; Jiang, L.P.; Qin, X.J.; Liu, Y.P.; Shen, Q.S.; Chen, Y.B.; Luo, X.D. Indole Alkaloids with New Skeleton Activating Neural Stem Cells. Org. Lett. 2014, 16, 5808–5811. [Google Scholar] [CrossRef]
- Yang, X.W.; Luo, X.D.; Lunga, P.K.; Zhao, Y.L.; Qin, X.J.; Chen, Y.Y.; Liu, L.; Li, X.N.; Liu, Y.P. Scholarisines H–O, Novel Indole Alkaloid Derivatives from Long-Term Stored Alstonia scholaris. Tetrahedron 2015, 71, 3694–3698. [Google Scholar] [CrossRef]
- Yang, X.W.; Song, C.W.; Zhang, Y.; Khan, A.; Jiang, L.P.; Chen, Y.B.; Liu, Y.P.; Luo, X.D. Alstoscholarisines F and G, Two Unusual Monoterpenoid Indole Alkaloids from the Leaves of Alstonia scholaris. Tetrahedron Lett. 2015, 56, 6715–6718. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Yao, X.J.; Liu, L.; Bai, L.P.; Jiang, Z.H. Alistonitrine A, a Caged Monoterpene Indole Alkaloid from Alstonia scholaris. Org. Lett. 2014, 16, 1080–1083. [Google Scholar] [CrossRef]
- Feng, T.; Cai, X.H.; Du, Z.Z.; Luo, X.D. Iridoids from the Bark of Alstonia scholaris. Helv. Chim. Acta 2008, 91, 2247–2251. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, T.; Cai, X.-H.; Luo, X.-D. A New C13-Norisoprenoid from Leaves of Alstonia scholaris. Chin. J. Nat. Med. 2009, 7, 21–23. [Google Scholar] [CrossRef]
- Taek, M.M.; Tukan, G.D.; Prajogo, B.E.W.; Agil, M. Antiplasmodial Activity and Phytochemical Constituents of Selected Antimalarial Plants Used by Native People in West Timor Indonesia. Turk. J. Pharm. Sci. 2021, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, S.; Tambunan, R.M.; Farida, Y.; Sandhiutami, N.M.D.; Dewi, R.M. Phytochemical Screening and Antimalarial Activity of Some Plants Traditionally Used in Indonesia. Asian Pac. J. Trop. Dis. 2015, 5, 454–457. [Google Scholar] [CrossRef]
- Ouattara, Y.; Sanon, S.; Traoré, Y.; Mahiou, V.; Azas, N.; Sawadogo, L. Antimalarial Activity of Swartzia Madagascariensis Desv. (Leguminosae), Combretum Glutinosum Guill. & Perr. (Combretaceae) and Tinospora Bakis Miers. (Menispermaceae), Burkina Faso Medicinal Plants. Afr. J. Tradit. Complement. Altern. Med. 2005, 3, 75–81. [Google Scholar] [CrossRef]
- Keawpradub, N.; Kirby, G.C.; Steele, J.C.P.; Houghton, P.J. Antiplasmodial Activity of Extracts and Alkaloids of Three Alstonia Species from Thailand. Planta Med. 1999, 65, 690–694. [Google Scholar] [CrossRef]
- Surur, A.S.; Huluka, S.A.; Mitku, M.L.; Asres, K. Indole: The After Next Scaffold of Antiplasmodial Agents? Drug Des. Dev. Ther. 2020, 14, 4855. [Google Scholar] [CrossRef]
- Muñoz, V.; Sauvain, M.; Bourdy, G.; Callapa, J.; Bergeron, S.; Rojas, I.; Bravo, J.A.; Balderrama, L.; Ortiz, B.; Gimenez, A.; et al. A Search for Natural Bioactive Compounds in Bolivia through a Multidisciplinary Approach. Part I. Evaluation of the Antimalarial Activity of Plants Used by the Chacobo Indians. J. Ethnopharmacol. 2000, 69, 127–137. [Google Scholar] [CrossRef]
- Intan, P.; Winarno, M.; Prihartini, N. Efek Ekstrak Campuran Kulit Batang Pulai (Alstonia scholaris) Dan Meniran (Phyllanthus niruri) Pada Mencit Swiss Webster Yang Diinfeksi Plasmodium berghei. J. Kefarmasian Indones. 2016, 6, 79–88. [Google Scholar] [CrossRef]
- Bello, I.; Bakkouri, A.; Tabana, Y.; Al-Hindi, B.; Al-Mansoub, M.; Mahmud, R.; Asmawi, M.Z. Acute and Sub-Acute Toxicity Evaluation of the Methanolic Extract of Alstonia scholaris Stem Bark. Med. Sci. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Jagetia, G.C.; Ulloor, J.N.; Baliga, M.P.; Venkatesh, P.; Reddy, R.; Rao, K.V.N.M.; Baliga, B.S.; Devi, S.; Raju, S.K.; et al. The Evaluation of the Acute Toxicity and Long Term Safety of Hydroalcoholic Extract of Sapthaparna (Alstonia scholaris) in Mice and Rats. Toxicol. Lett. 2004, 151, 317–326. [Google Scholar] [CrossRef]
- OECD. OECD Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2022; ISBN 9789264071049. [Google Scholar]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P. In Vitro Antiplasmodial Activity of Chosen Terrestrial Medicinal Plants against Plasmodium falciparum. Asian Pac. J. Trop. Biomed. 2012, 2, 252–256. [Google Scholar] [CrossRef]
- Atanu, F.O.; Idih, F.M.; Nwonuma, C.O.; Hetta, H.F.; Alamery, S.; El-Saber Batiha, G. Evaluation of Antimalarial Potential of Extracts from Alstonia boonei and Carica papaya in Plasmodium berghei-Infected Mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 2599191. [Google Scholar] [CrossRef] [PubMed]
- Melariri, P.; Campbell, W.; Etusim, P.; Smith, P. Antiplasmodial Properties and Bioassay-Guided Fractionation of Ethyl Acetate Extracts from Carica papaya Leaves. J. Parasitol. Res. 2011, 2011, 104954. [Google Scholar] [CrossRef]
- Julianti, T.; De Mieri, M.; Zimmermann, S.; Ebrahimi, S.N.; Kaiser, M.; Neuburger, M.; Raith, M.; Brun, R.; Hamburger, M. HPLC-Based Activity Profiling for Antiplasmodial Compounds in the Traditional Indonesian Medicinal Plant Carica papaya L. J. Ethnopharmacol. 2014, 155, 426–434. [Google Scholar] [CrossRef]
- Teng, W.C.; Chan, W.; Suwanarusk, R.; Ong, A.; Ho, H.K.; Russell, B.; Rénia, L.; Koh, H.L. In Vitro Antimalarial Evaluations and Cytotoxicity Investigations of Carica papaya Leaves and Carpaine. Nat. Prod. Commun. 2019, 14, 33–36. [Google Scholar] [CrossRef]
- Zeleke, G.; Kebebe, D.; Mulisa, E.; Gashe, F. In Vivo Antimalarial Activity of the Solvent Fractions of Fruit Rind and Root of Carica papaya Linn (Caricaceae) against Plasmodium berghei in Mice. J. Parasitol. Res. 2017, 2017, 3121050. [Google Scholar] [CrossRef]
- Okpe, O.; Habila, N.; Ikwebe, J.; Upev, V.A.; Okoduwa, S.I.R.; Isaac, O.T. Antimalarial Potential of Carica papaya and Vernonia amygdalina in Mice Infected with Plasmodium berghei. J. Trop. Med. 2016, 2016, 8738972. [Google Scholar] [CrossRef]
- Kovendan, K.; Murugan, K.; Panneerselvam, C.; Aarthi, N.; Kumar, P.M.; Subramaniam, J.; Amerasan, D.; Kalimuthu, K.; Vincent, S. Antimalarial Activity of Carica papaya (Family: Caricaceae) Leaf Extract against Plasmodium falciparum. Asian Pac. J. Trop. Dis. 2012, 2, S306–S311. [Google Scholar] [CrossRef]
- Widyawaruyanti, A.; Asrory, M.; Ekasari, W.; Setiawan, D.; Radjaram, A.; Tumewu, L.; Hafid, A.F. In Vivo Antimalarial Activity of Andrographis paniculata Tablets. Procedia Chem. 2014, 13, 101–104. [Google Scholar] [CrossRef]
- Widyawaruyanti, A.; Jonosewojo, A.; Ilmi, H.; Tumewu, L.; Imandiri, A.; Widiastuti, E.; Dachliyati, L.; Budiman, M.F.; Setyawan, D.; Hafid, A.F.; et al. Safety Evaluation of an Antimalarial Herbal Product from Andrographis paniculata (AS201-01) in Healthy Volunteers. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Zein, U.; Fitri, L.E.; Saragih, A. Comparative Study of Antimalarial Effect of Sambiloto (Andrographis paniculata) Extract, Chloroquine and Artemisinin and Their Combination against Plasmodium falciparum In-Vitro. Indones. J. Intern. Med. 2013, 45, 38–43. [Google Scholar]
- Makmur, T.; Siregar, F.A.; Siregar, S.; Lubis, I.A.; Bestari, R.; Zein, U. Open Clinical Trial of Sambiloto (Andrographis paniculata) Ethanolic Extract Capsules in Treatment of Malaria Patients in Batubara District, Indonesia. Med. Arch. 2022, 76, 419. [Google Scholar] [CrossRef]
- Mishra, K.; Dash, A.P.; Swain, B.K.; Dey, N. Anti-Malarial Activities of Andrographis paniculata and Hedyotis corymbosa Extracts and Their Combination with Curcumin. Malar. J. 2009, 8, 26. [Google Scholar] [CrossRef]
- Mishra, K.; Dash, A.P.; Dey, N. Andrographolide: A Novel Antimalarial Diterpene Lactone Compound from Andrographis paniculata and Its Interaction with Curcumin and Artesunate. J. Trop. Med. 2011, 2011, 579518. [Google Scholar] [CrossRef]
- Hassan, W.R.M.; Basir, R.; Ali; Embi, N. Sidek Anti-Malarial and Cytokine-Modulating Effects of Andrographolide in a Murine Model of Malarial Infection. Trop. Biomed. 2019, 36, 776–791. [Google Scholar]
- Rahman, N.N.N.A.; Furuta, T.; Kojima, S.; Takane, K.; Mohd, M.A. Antimalarial Activity of Extracts of Malaysian Medicinal Plants. J. Ethnopharmacol. 1999, 64, 249–254. [Google Scholar] [CrossRef]
- Sá, M.S.; De Menezes, M.N.; Krettli, A.U.; Ribeiro, I.M.; Tomassini, T.C.B.; Ribeiro Dos Santos, R.; De Azevedo, W.F.; Soares, M.B.P. Antimalarial Activity of Physalins B, D, F, and G. J. Nat. Prod. 2011, 74, 2269–2272. [Google Scholar] [CrossRef]
- World Flora Online. Carica papaya L. Available online: http://www.worldfloraonline.org/taxon/wfo-0000588009 (accessed on 6 December 2022).
- iNaturalist. Carica papaya L. Observed in Hong Kong by Viviang. Available online: https://www.inaturalist.org/observations/71054060 (accessed on 16 January 2023).
- iNaturalist. Carica papaya L. Observed in Cayman Islands by Caymanmatt. Available online: https://www.inaturalist.org/observations/108519374 (accessed on 16 January 2023).
- iNaturalist. Carica papaya L. Observed in United States of America by Ojos_y_manos. Available online: https://www.inaturalist.org/observations/83679263 (accessed on 16 January 2023).
- Aravind, G.; Bhowmik, D.; Duraviel, S.; Harish, G. Traditional and Medicinal Uses of Carica papaya. J. Med. Plants Stud. 2013, 1, 7–15. [Google Scholar]
- Ghaffarilaleh, V.; Fisher, D.; Henkel, R. Carica papaya Seed Extract Slows Human Sperm. J. Ethnopharmacol. 2019, 241, 111972. [Google Scholar] [CrossRef]
- Sarimole, E.; Martosupono, M.; Semangun, H.; Mangimbulude, J.C. Pengobatan Penyakit Malaria Dengan Menggunakan Beberapa Jenis Nabati Di Kabupaten Raja Ampat. In Raja Ampat and Future of Humanity (as a World Heritage), Proceedings of the Seminar Nasional Raja Ampat, Raja Ampat, Indonesia, 12–13 August 2014; Mangimbulude, J.C., Martosupono, M., Puspita, D., Nugroho, K.P.A.N., Eds.; Universitas Kristen Satya Wacana: Salatiga, Indonesia, 2014. [Google Scholar]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Khor, B.K.K.; Chear, N.J.Y.; Azizi, J.; Khaw, K.Y. Chemical Composition, Antioxidant and Cytoprotective Potentials of Carica papaya Leaf Extracts: A Comparison of Supercritical Fluid and Conventional Extraction Methods. Molecules 2021, 26, 1489. [Google Scholar] [CrossRef]
- Yanty, N.A.M.; Marikkar, J.M.N.; Nusantoro, B.P.; Long, K.; Ghazali, H.M. Physico-Chemical Characteristics of Papaya (Carica papaya L.) Seed Oil of the Hong Kong/Sekaki Variety. J. Oleo Sci. 2014, 63, 885–892. [Google Scholar] [CrossRef]
- Kumaratilake, L.M.; Ferrante, A.; Robinson, B.S.; Jaeger, T.; Poulos, A. Enhancement of Neutrophil-Mediated Killing of Plasmodium falciparum Asexual Blood Forms by Fatty Acids: Importance of Fatty Acid Structure. Infect. Immun. 1997, 65, 4152. [Google Scholar] [CrossRef]
- Uzor, P.F. Alkaloids from Plants with Antimalarial Activity: A Review of Recent Studies. Evid.-Based Complement. Altern. Med. 2020, 2020, 8749083. [Google Scholar] [CrossRef]
- Afzan, A.; Abdullah, N.R.; Halim, S.Z.; Rashid, B.A.; Semail, R.H.R.; Abdullah, N.; Jantan, I.; Muhammad, H.; Ismail, Z. Repeated Dose 28-Days Oral Toxicity Study of Carica papaya L. Leaf Extract in Sprague Dawley Rats. Molecules 2012, 17, 4326. [Google Scholar] [CrossRef]
- Ismail, Z.; Halim, S.Z.; Abdullah, N.R.; Afzan, A.; Abdul Rashid, B.A.; Jantan, I. Safety Evaluation of Oral Toxicity of Carica papaya Linn. Leaves: A Subchronic Toxicity Study in Sprague Dawley Rats. Evid.-Based Complement. Alternat. Med. 2014, 2014, 741470. [Google Scholar] [CrossRef]
- Lim, X.Y.; Chan, J.S.W.; Japri, N.; Lee, J.C.; Tan, T.Y.C. Carica Papaya L. Leaf: A Systematic Scoping Review on Biological Safety and Herb-Drug Interactions. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5511221. [Google Scholar] [CrossRef]
- Nghonjuyi, N.W.; Tiambo, C.K.; Taïwe, G.S.; Toukala, J.P.; Lisita, F.; Juliano, R.S.; Kimbi, H.K. Acute and Sub-Chronic Toxicity Studies of Three Plants Used in Cameroonian Ethnoveterinary Medicine: Aloe vera (L.) Burm. f. (Xanthorrhoeaceae) Leaves, Carica papaya L. (Caricaceae) Seeds or Leaves, and Mimosa pudica L. (Fabaceae) Leaves in Kabir Chicks. J. Ethnopharmacol. 2016, 178, 40–49. [Google Scholar] [CrossRef]
- Shrivastava, S.; Ansari, A.S.; Lohiya, N.K. Fertility, Developmental Toxicity and Teratogenicity in Albino Rats Treated with Methanol Sub-Fraction of Carica papaya Seeds. Indian J. Pharmacol. 2011, 43, 419–423. [Google Scholar] [CrossRef]
- World Flora Online. Andrographis paniculata (Burm.f.) Wall. Available online: http://www.worldfloraonline.org/taxon/wfo-0000534069 (accessed on 8 December 2022).
- Kumar, A.; Dora, J.; Singh, A.; Tripathi, R. A review on king of bitter (kalmegh). Int. J. Res. Pharm. Chem. 2012, 2, 116–124. [Google Scholar]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Qrimida, A.M.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. Ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef]
- Akbar, S. Andrographis paniculata: A Review of Pharmacological Activities and Clinical Effects. Altern. Med. Rev. 2011, 16, 66–77. [Google Scholar]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Rahman, K.M.H. Andrographis paniculata (Burm. f.) Wall. Ex Nees: A Review of Ethnobotany, Phytochemistry, and Pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef]
- iNaturalist. Andrographis paniculata (Burm.Fil.) Nees Observed in Thailand by Pakster. Available online: https://www.inaturalist.org/observations/114184625 (accessed on 18 January 2023).
- iNaturalist. Andrographis paniculata (Burm.Fil.) Nees Observed in India by Purab Chowdhury. Available online: https://www.inaturalist.org/observations/103810615 (accessed on 18 January 2023).
- Anju, D.; Jugnu, G.; Kavita, S.; Arun, N.; Sandeep, D. A Review on Medicinal Prospectives of Andrographis paniculata Nees. J. Pharm. Sci. Innov. 2012, 1, 1–4. [Google Scholar]
- Ji, L.L.; Wang, Z.; Dong, F.; Zhang, W.B.; Wang, Z.T. Andrograpanin, a Compound Isolated from Anti-Inflammatory Traditional Chinese Medicine Andrographis paniculata, Enhances Chemokine SDF-1alpha-Induced Leukocytes Chemotaxis. J. Cell. Biochem. 2005, 95, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-ard, P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Sato, M.; Ueno, A.; Nishi, K. Flavonoids from Andrographis paniculata. Chem. Pharm. Bull. 1987, 35, 4429–4435. [Google Scholar] [CrossRef]
- Xu, C.; Chou, G.X.; Wang, C.H.; Wang, Z.T. Rare Noriridoids from the Roots of Andrographis paniculata. Phytochemistry 2012, 77, 275–279. [Google Scholar] [CrossRef] [PubMed]
- My, N.T.T.; Hanh, T.T.H.; Cham, P.T.; Cuong, N.X.; Huong, T.T.; Quang, T.H.; Nam, N.H.; Minh, C. Van Andropaniosides A and B, Two New Ent-Labdane Diterpenoid Glucosides from Andrographis paniculata. Phytochem. Lett. 2020, 35, 37–40. [Google Scholar] [CrossRef]
- Shen, Y.H.; Li, R.T.; Xiao, W.L.; Gang-Xu; Lin, Z.W.; Zhao, Q.S.; Sun, H.D. Ent-Labdane Diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006, 69, 319–322. [Google Scholar] [CrossRef]
- Wang, G.C.; Wang, Y.; Williams, I.D.; Sung, H.H.Y.; Zhang, X.Q.; Zhang, D.M.; Jiang, R.W.; Yao, X.S.; Ye, W.C. Andrographolactone, a Unique Diterpene from Andrographis paniculata. Tetrahedron Lett. 2009, 50, 4824–4826. [Google Scholar] [CrossRef]
- Pramanick, S.; Banerjee, S.; Achari, B.; Das, B.; Sen, A.K.; Mukhopadhyay, S.; Neuman, A.; Prangé, T. Andropanolide and Isoandrographolide, Minor Diterpenoids from Andrographis paniculata: Structure and X-Ray Crystallographic Analysis. J. Nat. Prod. 2006, 69, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A. Cell Differentiation-Inducing Diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994, 42, 1216–1225. [Google Scholar] [CrossRef]
- Allison, A.J.; Butcher, D.N.; Connolly, J.D.; Overton, K.H. Paniculides A, B, and C, Bisabolenoid Lactones from Tissue Cultures of Andrographis paniculata. Chem. Commun. 1968, 23, 1493. [Google Scholar] [CrossRef]
- Wu, T.S.; Chern, H.J.; Damu, A.G.; Kuo, P.C.; Su, C.R.; Lee, E.J.; Teng, C.M. Flavonoids and Ent-Labdane Diterpenoids from Andrographis paniculata and Their Antiplatelet Aggregatory and Vasorelaxing Effects. J. Asian Nat. Prod. Res. 2008, 10, 17–24. [Google Scholar] [CrossRef]
- Xu, C.; WAng, Z.-T. Chemical Constituents from Roots of Andrographis paniculata. Yao Xue Xue Bao 2011, 46, 317–321. [Google Scholar]
- Chua, L.S.; Yap, K.C.; Jaganath, I.B. Comparison of Total Phenolic Content, Scavenging Activity and HPLC-ESI-MS/MS Profiles of Both Young and Mature Leaves and Stems of Andrographis paniculata. Nat. Prod. Commun. 2013, 8, 1725–1729. [Google Scholar] [CrossRef]
- Koteswara Rao, Y.; Vimalamma, G.; Venkata Rao, C.; Tzeng, Y.M. Flavonoids and Andrographolides from Andrographis paniculata. Phytochemistry 2004, 65, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, B.; Juma, E.; Obonyo, C.; Jullien, V.; Carn, G.; Vaillant, M.; Taylor, W.R.J.; Kiechel, J.R. Fixed Dose Artesunate Amodiaquine—A Phase IIb, Randomized Comparative Trial with Non-Fixed Artesunate Amodiaquine. Malar. J. 2014, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Worasuttayangkurn, L.; Nakareangrit, W.; Kwangjai, J.; Sritangos, P.; Pholphana, N.; Watcharasit, P.; Rangkadilok, N.; Thiantanawat, A.; Satayavivad, J. Acute Oral Toxicity Evaluation of Andrographis paniculata-Standardized First True Leaf Ethanolic Extract. Toxicol. Rep. 2019, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, C.V.; Thiyagarajan, P.; Sundarajan, K.; Goudar, K.S.; Deepak, M.; Murali, B.; Joshua Allan, J.; Agarwal, A. Evaluation of the Genotoxic Potential and Acute Oral Toxicity of Standardized Extract of Andrographis paniculata (KalmColdTM). Food Chem. Toxicol. 2009, 47, 1892–1902. [Google Scholar] [CrossRef]
- Burgos, R.A.; Caballero, E.E.; Sánchez, N.S.; Schroeder, R.A.; Wikman, G.K.; Hancke, J.L. Testicular Toxicity Assesment of Andrographis paniculata Dried Extract in Rats. J. Ethnopharmacol. 1997, 58, 219–224. [Google Scholar] [CrossRef]
- Al Batran, R.; Al-Bayaty, F.; Al-Obaidi, M.M.J.; Abdulla, M.A. Acute Toxicity and the Effect of Andrographolide on Porphyromonas Gingivalis-Induced Hyperlipidemia in Rats. Biomed. Res. Int. 2013, 2013, 594012. [Google Scholar] [CrossRef]
- Bothiraja, C.; Pawar, A.P.; Shende, V.S.; Joshi, P.P. Acute and Subacute Toxicity Study of Andrographolide Bioactive in Rodents: Evidence for the Medicinal Use as an Alternative Medicine. Comp. Clin. Pathol. 2013, 22, 1123–1128. [Google Scholar] [CrossRef]
- World Flora Online. Physalis minima L. Available online: http://www.worldfloraonline.org/taxon/wfo-0001024837 (accessed on 8 December 2022).
- Patel, T.; Shah, K.; Jiwan, K.; Shrivastava, N. Study on the Antibacterial Potential of Physalis minima Linn. Indian J. Pharm. Sci. 2011, 73, 111. [Google Scholar] [CrossRef]
- Alegantina, S.; Setyorini, H.A.; Oktoberia, I.S.; Winarsih; Aini, N. Physalis minima L. Profile from Various Ethnics in 9 (Nine) Provinces Indonesia by HPLCand Chemometric. Media Penelit. Dan Pengemb. Kesehat. 2021, 31, 17–26. [Google Scholar]
- Khan, M.A.; Khan, H.; Khan, S.; Mahmood, T.; Khan, P.M.; Jabar, A. Anti-Inflammatory, Analgesic and Antipyretic Activities of Physalis minima Linn. J. Enzym. Inhib. Med. Chem. 2009, 24, 632–637. [Google Scholar] [CrossRef]
- Habib, A. Wildgooseberry (Physalis minima). Available online: https://www.inaturalist.org/observations/23892499#data_quality_assessment (accessed on 9 December 2022).
- Shil, D.; Sarma, T.; Roy, S.D.; Chakraborty, J.; Das, S.R.C.; Das, T.; Rohman, T.A. Exploring Quality Control Standards and Potential Antibacterial Property of Different Extracts of the Root of Physalis minima L. Int. J. Res. Pharm. Sci. 2021, 12, 2350–2360. [Google Scholar] [CrossRef]
- Chothani, D.L.; Vaghasiya, H.U. A Phyto-Pharmacological Overview on Physalis minima Linn. Indian J. Nat. Prod. Resour. 2012, 3, 477–482. [Google Scholar]
- Parkash, V.; Aggarwal, A. Traditional Uses of Ethnomedicinal Plants of Lower Foot-Hills of Himachal Pradesh-I. Indian J. Tradit. Knowl. 2010, 9, 519–521. [Google Scholar]
- Zakaria, M.; Mohd, M.A. Traditional Malay Medicinal Plants; Institut Terjemahan & Buku Malaysia: Kuala Lumpur, Malaysia, 2015; ISBN 978-9-83-068385-0. [Google Scholar]
- Sinha, S.C.; Ray, A.B. Chemical Constituents of Physalis minima Var. Indica. J. Indian Chem. Soc. 1988, LXV, 740–741. [Google Scholar] [CrossRef]
- Ooi, K.L.; Tengku Muhammad, T.S.; Sulaiman, S.F. Physalin F from Physalis minima L. Triggers Apoptosis-Based Cytotoxic Mechanism in T-47D Cells through the Activation Caspase-3- and c-Myc-Dependent Pathways. J. Ethnopharmacol. 2013, 150, 382–388. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Yousaf, S.; Ahmed, S.; Samreen; Yasmeen, K. Atta-ur-Rahmang Antileishmanial Physalins from Physalis minima. Chem. Biodivers. 2005, 2, 1164–1173. [Google Scholar] [CrossRef]
- Sen, G.; Pathak, H.D. Physalin L, a 13,14-Seco-16,24 Cyclosteroid from Physalis minima. Phytochemistry 1995, 39, 1245–1246. [Google Scholar] [CrossRef]
- Misra, L.N.; Lal, P.; Kumar, D. Isolation and Characterization of Steroids of Nutraceutical Value in Physalis minima. J. Food Sci. Nutr. 2006, 11, 133–139. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Cojocaru, M.; Sinha, S.C.; Saha, M.; Bagchi, A.; Ali, A.; Ray, A.B. Withaminimin, a Withanolide from Physalis minima. Phytochemistry 1987, 26, 1801–1804. [Google Scholar] [CrossRef]
- Basey, K.; McGaw, B.A.; Woolley, J.G. Phygrine, an Alkaloid from Physalis Species. Phytochemistry 1992, 31, 4173–4176. [Google Scholar] [CrossRef]
- Ser, N.A. Flavonoids from Physalis minima. Phytochemistry 1988, 27, 3708–3709. [Google Scholar] [CrossRef]
- Lusakibanza, M.; Mesia, G.; Tona, G.; Karemere, S.; Lukuka, A.; Tits, M.; Angenot, L.; Frédérich, M. In Vitro and In Vivo Antimalarial and Cytotoxic Activity of Five Plants Used in Congolese Traditional Medicine. J. Ethnopharmacol. 2010, 129, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Arruda, J.C.C.; Rocha, N.C.; Santos, E.G.; Ferreira, L.G.B.; Bello, M.L.; Penido, C.; Costa, T.E.M.M.; Santos, J.A.A.; Ribeiro, I.M.; Tomassini, T.C.B.; et al. Physalin Pool from Physalis angulata L. Leaves and Physalin D Inhibit P2X7 Receptor Function in Vitro and Acute Lung Injury in Vivo. Biomed. Pharmacother. 2021, 142, 112006. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Levano-Garcia, J.; Dluzewski, A.R.; Markus, R.P.; Garcia, C.R.S. Purinergic Signalling Is Involved in the Malaria Parasite Plasmodium falciparum Invasion to Red Blood Cells. Purinergic Signal. 2010, 6, 365–372. [Google Scholar] [CrossRef]
- Zafirah Ismail, N.; Arsad, H.; Afiqah Lokman, N.; Izzati Jaafar, N.; Hasyimah Haron, N. Preliminary Acute Oral Toxicity Study of The Water Extract of Physalis minima Leaves. J. Biol. Sci. Opin. 2018, 6, 111–115. [Google Scholar] [CrossRef]
- Soeharto, S.; Nugrahenny, D.; Permatasari, N.; Mayangsari, E. Subchronic Toxicity of the Physalis minima Leaves. Res. J. Life Sci. 2018, 5, 77–82. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indradi, R.B.; Muhaimin, M.; Barliana, M.I.; Khatib, A. Potential Plant-Based New Antiplasmodial Agent Used in Papua Island, Indonesia. Plants 2023, 12, 1813. https://doi.org/10.3390/plants12091813
Indradi RB, Muhaimin M, Barliana MI, Khatib A. Potential Plant-Based New Antiplasmodial Agent Used in Papua Island, Indonesia. Plants. 2023; 12(9):1813. https://doi.org/10.3390/plants12091813
Chicago/Turabian StyleIndradi, Raden Bayu, Muhaimin Muhaimin, Melisa Intan Barliana, and Alfi Khatib. 2023. "Potential Plant-Based New Antiplasmodial Agent Used in Papua Island, Indonesia" Plants 12, no. 9: 1813. https://doi.org/10.3390/plants12091813
APA StyleIndradi, R. B., Muhaimin, M., Barliana, M. I., & Khatib, A. (2023). Potential Plant-Based New Antiplasmodial Agent Used in Papua Island, Indonesia. Plants, 12(9), 1813. https://doi.org/10.3390/plants12091813