Metabolomics Reveals Rubiadin Accumulation and the Effects of Methyl Jasmonate Elicitation in Damnacanthus major Calli
Abstract
:1. Introduction
2. Results and Discussion
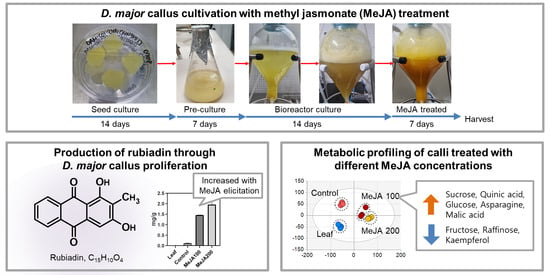
2.1. Effects of Methyl Jasmonate on Callus Proliferation
2.2. Rubiadin Production through Callus Suspension Cultures
2.3. Principal Component Analysis and Metabolite–Metabolite Correlations
2.4. Major Metabolite Differences in Response to MeJA Treatment and Concentrations
3. Materials and Methods
3.1. Callus Production and Elicitation
3.2. Preparation of Plant Materials
3.3. Extraction and Analysis of Rubiadin
3.4. Extraction and Analysis of Phenolic Compounds
3.5. Extraction and Analysis of Low-Molecular Hydrophilic Compounds
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jang, Y.-J.; Park, J.-S.; Lee, J.-S.; Lee, J.-Y.; Choi, B.-H. Re-Examination of the Vascular Plants on Hongdo Island, Korea. Korean J. Plant Taxon. 2021, 51, 205–249. [Google Scholar] [CrossRef]
- Tao, C.; Hua, Z.; Jiarui, C.; Taylor, C.M.; Ehrendorfer, F.; Lantz, H.; Funston, A.M.; Puff, C. Rubiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MI, USA, 2011; Volume 19, pp. 654–656. [Google Scholar]
- Lou, S.; Li, H.; Qin, X.; Huang, Z.; Jiang, S.; Huang, X. The Complete Chloroplast Genome of Damnacanthus indicus CF Gaertn. (Rubiaceae). Mitochondrial DNA Part B 2021, 6, 1251–1252. [Google Scholar] [CrossRef]
- Han, E.-K.; Cho, W.-B.; Tamaki, I.; Choi, I.-S.; Lee, J.-H. Comparative Mitogenomic Analysis Reveals Gene and Intron Dynamics in Rubiaceae and Intra-Specific Diversification in Damnacanthus indicus. Int. J. Mol. Sci. 2021, 22, 7237. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, X.; Wang, Y.; Nong, B.; Tang, C.; Lu, J.; Zhao, F. Analysis and Study on Quality Control of Yao Medicine Damnacanthus macrophyllus Based on Methods in Chinese Pharmacopoeia. Med. Plant 2021, 12, 40–43. [Google Scholar] [CrossRef]
- Suksungworn, R.; Duangsrisai, S. Phytochemical Contents and Antioxidant Activity of Medicinal Plants from the Rubiaceae Family in Thailand. Plant Sci. Today 2021, 8, 24–31. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Anthraquinone Production from Cell and Organ Cultures of Rubia Species: An Overview. Metabolites 2022, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Das, B.P.; Ghosh, A. Medicinal Properties of Phyto-Constituents from Rubiaceae Family of Assam: Critical Review. Int. J. Appl. Chem. Biol. Sci. 2020, 1, 1–25. [Google Scholar]
- D’Amelia, V.; Docimo, T.; Crocoll, C.; Rigano, M.M. Specialized Metabolites and Valuable Molecules in Crop and Medicinal Plants: The Evolution of their use and Strategies for their Production. Genes 2021, 12, 936. [Google Scholar] [CrossRef]
- Benjamin, E.D.; Ishaku, G.A.; Peingurta, F.A.; Afolabi, A.S. Callus Culture for the Production of Therapeutic Compounds. Am. J. Plant Biol. 2019, 4, 76–84. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tiss. Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; IntechOpen Limited: London, UK, 2016; pp. 247–277. [Google Scholar] [CrossRef]
- Komaraiah, P.; Kishor, P.K.; Carlsson, M.; Magnusson, K.; Mandenius, C. Enhancement of Anthraquinone Accumulation in Morinda Citrifolia Suspension Cultures. Plant Sci. 2005, 168, 1337–1344. [Google Scholar] [CrossRef]
- Khoo, S.H.G.; Al-Rubeai, M. Metabolomics as a Complementary Tool in Cell Culture. Biotechnol. Appl. Biochem. 2007, 47, 71–84. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites Against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Nabi, N.; Singh, S.; Saffeullah, P. Responses of in Vitro Cell Cultures to Elicitation: Regulatory Role of Jasmonic Acid and Methyl Jasmonate: A Review. Vitr. Cell. Dev. Biol. Plant 2021, 57, 341–355. [Google Scholar] [CrossRef]
- Chaudhary, G.; Dantu, P.K. Evaluation of Callus Browning and Develop a Strategically Callus Culturing of Boerhaavia diffusa L. J. Plant Develop. 2015, 22, 47–58. [Google Scholar]
- Saeed, S.; Ali, H.; Khan, T.; Kayani, W.; Khan, M.A. Impacts of Methyl Jasmonate and Phenyl Acetic Acid on Biomass Accumulation and Antioxidant Potential in Adventitious Roots of Ajuga bracteosa Wall Ex Benth., a High Valued Endangered Medicinal Plant. Physiol. Mol. Biol. Plants 2017, 23, 229–237. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of Salicylic Acid and Methyl Jasmonate in the Production of Phenolic Compounds in Plant Cell Suspension Cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The Effects of Jasmonic Acid and Methyl Jasmonate on Rosmarinic Acid Production in Mentha× Piperita Cell Suspension Cultures. Plant Cell Tiss. Organ Cult. 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Watroly, M.N.; Sekar, M.; Fuloria, S.; Gan, S.H.; Jeyabalan, S.; Wu, Y.S.; Subramaniyan, V.; Sathasivam, K.V.; Ravi, S.; Mat Rani, N.N.I. Chemistry, Biosynthesis, Physicochemical and Biological Properties of Rubiadin: A Promising Natural Anthraquinone for New Drug Discovery and Development. Drug Des. Devel. Ther. 2021, 15, 4527–4549. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Yu, X.; Cao, F.; Cai, X.; Chen, P.; Li, M.; Feng, Y.; Li, H.; Wang, X. Pharmacokinetics of Anthraquinones from Medicinal Plants. Front. Pharmacol. 2021, 12, 638993. [Google Scholar] [CrossRef] [PubMed]
- Perassolo, M.; Cardillo, A.B.; Mugas, M.L.; Montoya, S.C.N.; Giulietti, A.M.; Talou, J.R. Enhancement of Anthraquinone Production and Release by Combination of Culture Medium Selection and Methyl Jasmonate Elicitation in Hairy Root Cultures of Rubia tinctorum. Ind. Crops Prod. 2017, 105, 124–132. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Y.; Chen, L.; Yi, S.; Tan, N. De Novo Transcriptome Analysis Reveals Putative Genes Involved in Anthraquinone Biosynthesis in Rubia yunnanensis. Genes 2022, 13, 521. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, J.-E.; Murthy, H.N.; Kim, J.-Y.; Kim, M.-J.; Jeong, T.-K.; Park, S.-Y. Production of Secondary Metabolites from Cell Cultures of Sageretia thea (Osbeck) MC Johnst. using Balloon-Type Bubble Bioreactors. Plants 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C. Exploring Plant Tissue Culture to Improve the Production of Phenolic Compounds: A Review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic Profiles of Cultivated, in Vitro Cultured and Commercial Samples of Melissa officinalis L. Infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar]
- Hendriks, M.M.; van Eeuwijk, F.A.; Jellema, R.H.; Westerhuis, J.A.; Reijmers, T.H.; Hoefsloot, H.C.; Smilde, A.K. Data-Processing Strategies for Metabolomics Studies. TrAC Trends Anal. Chem. 2011, 30, 1685–1698. [Google Scholar] [CrossRef]
- Singh, S.; Tanwer, B.S.; Khan, M. Callus Induction and in Vivo and in Vitro Comparative Study of Primary Metabolites of Withania somnifera. Adv. Appl. Sci. Res. 2011, 2, 47–52. [Google Scholar]
- Al-Jibouri, A.M.J.; Abed, A.S.; Ali, A.A.; Majeed, D.M. Improvement of Phenols Production by Amino Acids in Callus Cultures of Verbascum thapsus L. Am. J. Plant Sci. 2016, 7, 84–91. [Google Scholar] [CrossRef]
- Kim, T.J.; Hyeon, H.; Park, N.I.; Yi, T.G.; Lim, S.-H.; Park, S.-Y.; Ha, S.-H.; Kim, J.K. A High-Throughput Platform for Interpretation of Metabolite Profile Data from Pepper (Capsicum) Fruits of 13 Phenotypes Associated with Different Fruit Maturity States. Food Chem. 2020, 331, 127286. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arias, D.; Garcia-Machado, F.J.; Morales-Sierra, S.; Luis, J.C.; Suarez, E.; Hernández, M.; Valdés, F.; Borges, A.A. Lettuce Plants Treated with L-Pyroglutamic Acid Increase Yield Under Water Deficit Stress. Environ. Exp. Bot. 2019, 158, 215–222. [Google Scholar] [CrossRef]
- Zarbakhsh, S.; Shahsavar, A.R. Exogenous Γ-Aminobutyric Acid Improves the Photosynthesis Efficiency, Soluble Sugar Contents, and Mineral Nutrients in Pomegranate Plants Exposed to Drought, Salinity, and Drought-Salinity Stresses. BMC Plant Biol. 2023, 23, 543. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Lim, S.-H.; Ha, S.-H.; Yeo, Y.; Park, W.T.; Kwon, D.Y.; Park, S.U.; Kim, J.K. Metabolite Profiling Approach Reveals the Interface of Primary and Secondary Metabolism in Colored Cauliflowers (Brassica oleracea L. ssp. Botrytis). J. Agric. Food Chem. 2013, 61, 6999–7007. [Google Scholar] [CrossRef]
- Park, H.-E.; Kim, Y.J.; Do, K.-H.; Kim, J.K.; Ham, J.-S.; Lee, W.-K. Effects of Queso Blanco Cheese Containing Bifidobacterium longum KACC 91563 on the Intestinal Microbiota and Short Chain Fatty Acid in Healthy Companion Dogs. Korean J. Food Sci. Anim. Resour. 2018, 38, 1261. [Google Scholar] [CrossRef]
- Andi, S.A.; Gholami, M.; Ford, C.M.; Maskani, F. The Effect of Light, Phenylalanine and Methyl Jasmonate, Alone or in Combination, on Growth and Secondary Metabolism in Cell Suspension Cultures of Vitis Vinifera. J. Photochem. Photobiol. B Biol. 2019, 199, 111625. [Google Scholar] [CrossRef]
- Liu, Z.; Mohsin, A.; Wang, Z.; Zhu, X.; Zhuang, Y.; Cao, L.; Guo, M.; Yin, Z. Enhanced Biosynthesis of Chlorogenic Acid and its Derivatives in Methyl-Jasmonate-Treated Gardenia jasminoides Cells: A Study on Metabolic and Transcriptional Responses of Cells. Front. Bioeng. Biotechnol. 2021, 8, 604957. [Google Scholar] [CrossRef]
- Kdhim, S.J.; Jassim, E.H.; Salih, H.H. Effect of Salicylic Acid on Increasing of some Phenolic Acid and Flavonoids in Coriandrum sativum Callus. Plant Arch. 2020, 20, 3007–3014. [Google Scholar]
- Lahuta, L.B.; Zalewski, K.; Głowacka, K.; Nitkiewicz, B.; Amarowicz, R. Effect of Methyl Jasmonate on Carbohydrate Composition, A-Amylase Activity and Growth of Triticale (Triticosecale witmmack) Seedlings. J. Agr. Sci. Technol. 2018, 19, 1127–1137. [Google Scholar]
- Larronde, F.; Krisa, S.; Decendit, A.; Cheze, C.; Deffieux, G.; Mérillon, J. Regulation of Polyphenol Production in Vitis Vinifera Cell Suspension Cultures by Sugars. Plant Cell Rep. 1998, 17, 946–950. [Google Scholar] [CrossRef]
- Benevenuto, R.F.; Seldal, T.; Hegland, S.J.; Rodriguez-Saona, C.; Kawash, J.; Polashock, J. Transcriptional Profiling of Methyl Jasmonate-Induced Defense Responses in Bilberry (Vaccinium myrtillus L.). BMC Plant Biol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Lee, J.-E.; Cho, Y.U.; Kim, K.H.; Lee, D.Y. Distinctive Metabolomic Responses of Chlamydomonas reinhardtii to the Chemical Elicitation by Methyl Jasmonate and Salicylic Acid. Process Biochem. 2016, 51, 1147–1154. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.-H.; Park, S.U. Metabolomics Analysis and Biosynthesis of Rosmarinic Acid in Agastache rugosa Kuntze Treated with Methyl Jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Feng, Q.; Sun, H.; Zhang, X.; Li, X.; Li, J.; Gao, J. Response of Bioactive Metabolite and Biosynthesis Related Genes to Methyl Jasmonate Elicitation in Codonopsis pilosula. Molecules 2019, 24, 533. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological Aspects of Raffinose Family Oligosaccharides in Plants: Protection Against Abiotic Stress. Plant Biol. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Wu, Y.; Zhou, Y.; Jiang, Y.; Zhang, L. Effect of Elicitors on the Metabolites in the Suspension Cell Culture of Salvia miltiorrhiza Bunge. Physiol. Mol. Biol. Plants 2019, 25, 229–242. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Chowdhury, K.; Boroujerdi, A.; El-Bakry, A. Effect of Stress Hormones on the Metabolome of a Suspension Culture of the Aromatic Medicinal Plant Cymbopogon schoenanthus subsp. proximus. Plant Cell Tiss. Organ Cult. 2023, 155, 137–163. [Google Scholar] [CrossRef]
- Madhloom, A.A.A.; Abd-Al-Hussan, G.F.; Kadhim, N.J.; Abass, M.C.; Header, H.R.; Talib, A.K.; Al-Hussain, M.A.T.A.; Aziz, D.Z. The Interaction Effect of Salt Stress and Lysine Concentrations in some Properties of Fenugreek Callus. J. Phys. Conf. Ser. 2020, 1660, 012018. [Google Scholar] [CrossRef]
- Malarz, J.; Yudina, Y.V.; Stojakowska, A. Hairy Root Cultures as a Source of Phenolic Antioxidants: Simple Phenolics, Phenolic Acids, Phenylethanoids, and Hydroxycinnamates. Int. J. Mol. Sci. 2023, 24, 6920. [Google Scholar] [CrossRef]
- Classic Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Blömeke, B.; Poginsky, B.; Schmutte, C.; Marquardt, H.; Westendorf, J. Formation of Genotoxic Metabolites from Anthraquinone Glycosides, Present in Rubia tinctorum L. Mutat. Res. 1992, 265, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Jeong, C.-S.; Shin, Y.-K.; Park, K.-H.; Lee, W.-J.; Lee, E.-J.; Paek, K.-Y. Effects of Extraction Condition on Extraction Efficiency of Rubiadin in Adventitious Roots of Noni (Morinda citrifolia). Kor. J. Hort. Sci. Technol. 2010, 28, 685–690. [Google Scholar]
- Hyeon, H.; Jang, E.B.; Yoon, W.-J.; Lee, J.-D.; Hyun, H.B.; Jung, Y.-H.; Min, J.; Ham, Y.-M. Proliferation and Metabolic Profiling of Cynanchum wilfordii Adventitious Roots using Explants from Different Cultivation Methods. ACS Omega 2022, 7, 46756–46768. [Google Scholar] [CrossRef]
- Hyeon, H.; Hyun, H.B.; Go, B.; Kim, S.C.; Jung, Y.-H.; Ham, Y.-M. Profiles of Essential Oils and Correlations with Phenolic Acids and Primary Metabolites in Flower Buds of Magnolia heptapeta and Magnolia denudata var. purpurascens. Molecules 2021, 27, 221. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Huang, P.; Inoue, K. Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol. 2003, 44, 404–411. [Google Scholar] [CrossRef]
- Wang, J.; Qian, J.; Yao, L.; Lu, Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspensionculture of Hypericum perforatum. Bioresour. Bioprocess. 2015, 2, 5–13. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Chen, X.; Gong, X.; Wang, N.; Ma, L.; Qui, Y.; Wang, Y.; Feng, S. Effects of methyl jasmonate and abscisic acid on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tiss. Organ Cult. 2017, 130, 227–237. [Google Scholar] [CrossRef]
- Ali, M.B.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Differential responses of anti-oxidants enzymes, lipoxygenase activity, ascorbate content and the production of saponins in tissue cultured root of mountain Panax ginseng CA Mayer and Panax quinquefolium L. in bioreactor subjected to methyl jasmonate stress. Plant Sci. 2005, 169, 83–92. [Google Scholar] [CrossRef]
- Sampedro, M.A.S.; Tárrago, J.F.; Corchete, P. Yeast extract and methyl jasmonate-induced silymarin production in cell cultures of Silybum marianum (L.) Gaertn. J. Biotechnol. 2005, 119, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Z.J.; Xu, Y.; Qian, X.; Zhong, J.J. Efficient induction of ginsenoside biosynthesis and alteration of ginsenoside heterogeneity in cell cultures of Panax notoginseng by using chemically synthesized 2-hydroxyethyl jasmonate. Appl. Microbiol. Biotechnol. 2006, 70, 298–307. [Google Scholar] [CrossRef]
- Cho, H.Y.; Son, S.Y.; Rhee, H.S.; Yoon, S.Y.H.; Lee-Parsons, C.W.T.; Park, J.M. Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J. Biotechnol. 2008, 135, 117–122. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Palazon, J.; Eibl, R.; Cusido, R.M. Methyl jasmonate enhanced production of rosmarinic acid in cell cultures of Satureja khuzistanica in a bioreactor. Eng. Life Sci. 2016, 16, 740–749. [Google Scholar] [CrossRef]
- Cai, J.; Ma, Y.; Hu, P.; Zhang, Y.; Chen, J.; Li, X. Elicitation of furanocoumarins in Changium smyrnioides suspension cells. Plant Cell Tiss. Organ Cult. 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Sellapan, P.; Rohani, E.R.; Noor, N.M. Sesquiterpene production in methyl Jasmonate-induced Persicaria minor cell suspension culture. Sains Malays. 2018, 47, 3051–3059. [Google Scholar] [CrossRef]
- Liu, Z.B.; Chen, J.G.; Yin, Z.P.; Shangguan, X.C.; Peng, D.Y.; Lu, T.; Lin, P. Methyl jasmonate and salicylic acid elicitation increase content and yield of chlorogenic acid and its derivatives in Gardenia jasminoides cell suspension cultures. Plant Cell Tiss. Organ Cult. 2018, 134, 79–93. [Google Scholar] [CrossRef]
- Hao, Y.J.; Cui, X.H.; Li, J.R.; An, X.L.; Sun, H.D.; Piao, X.C.; Lian, M.L. Cell bioreactor culture of Orostachys cartilaginous A. Bor. and involvement of nitric oxide in methyl jasmonate-induced flavonoid synthesis. Acta Physiol. Plant. 2020, 42, 9. [Google Scholar] [CrossRef]





| (mg/g Dry Weight) | Leaves | Unelicited | Elicited | |
|---|---|---|---|---|
| Control | MeJA 100 | MeJA 200 | ||
| Rubiadin | * N.D. a | 0.121 ± 0.007 b | 1.458 ± 0.008 c | 1.961 ± 0.143 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeon, H.; Jang, E.B.; Kim, S.C.; Yoon, S.-A.; Go, B.; Lee, J.-D.; Hyun, H.B.; Ham, Y.-M. Metabolomics Reveals Rubiadin Accumulation and the Effects of Methyl Jasmonate Elicitation in Damnacanthus major Calli. Plants 2024, 13, 167. https://doi.org/10.3390/plants13020167
Hyeon H, Jang EB, Kim SC, Yoon S-A, Go B, Lee J-D, Hyun HB, Ham Y-M. Metabolomics Reveals Rubiadin Accumulation and the Effects of Methyl Jasmonate Elicitation in Damnacanthus major Calli. Plants. 2024; 13(2):167. https://doi.org/10.3390/plants13020167
Chicago/Turabian StyleHyeon, Hyejin, Eun Bi Jang, Sung Chun Kim, Seon-A Yoon, Boram Go, Jong-Du Lee, Ho Bong Hyun, and Young-Min Ham. 2024. "Metabolomics Reveals Rubiadin Accumulation and the Effects of Methyl Jasmonate Elicitation in Damnacanthus major Calli" Plants 13, no. 2: 167. https://doi.org/10.3390/plants13020167
APA StyleHyeon, H., Jang, E. B., Kim, S. C., Yoon, S. -A., Go, B., Lee, J. -D., Hyun, H. B., & Ham, Y. -M. (2024). Metabolomics Reveals Rubiadin Accumulation and the Effects of Methyl Jasmonate Elicitation in Damnacanthus major Calli. Plants, 13(2), 167. https://doi.org/10.3390/plants13020167