Host Plant Modulated Physio-Biochemical Process Enhances Adaptive Response of Sandalwood (Santalum album L.) under Salinity Stress
Abstract
:1. Introduction
2. Results
2.1. Morphological Parameters
2.2. Photosynthetic Pigments
2.3. Gas Exchange Parameters
2.4. Plant Water Relations
2.5. Ion Dynamics
2.6. Membrane Injury and Lipid Peroxidation
2.7. Antioxidant Enzyme
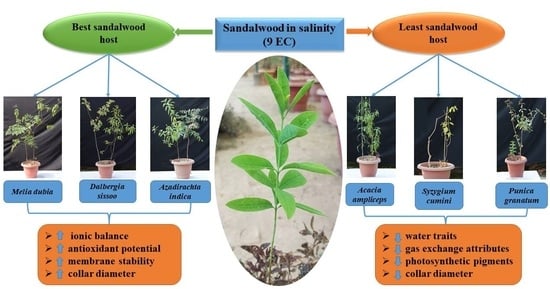
2.8. Host Plant Species Preference under Salinity Stress
3. Discussion
3.1. Photosynthetic Pigments
3.2. Gas Exchange Parameters
3.3. Plant Water Relations
3.4. Ion Dynamics
3.5. Membrane Injury and Lipid Peroxidation
3.6. Antioxidant Enzyme
4. Materials and Methods
4.1. Experimental Area and Planting Material
4.2. Experiment Description
4.3. Physiological Measurement
4.4. Ions Dynamics
4.5. Biochemical Traits
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabagh, A.E.L.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Sharma, D.K.; Singh, A. Current trends and emerging challenges in sustainable management of salt-affected soils: A critical appraisal. In Bioremediation of Salt Affected Soils: An Indian Perspective; Arora, S., Singh, A., Singh, Y., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–40. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- CSSRI. ICAR-Central Soil Salinity Research Institute Vision 2050; Indian Council of Agricultural Research: New Delhi, India, 2015. [Google Scholar]
- Sharma, D.K.; Thimmappa, K.; Chinchmalatpure, A.R.; Mandal, A.K.; Yadav, R.K.; Chaudhari, S.K.; Kumar, S.; Sikka, A.K. Assessment of Production and Monetary Losses from Salt Affected Soils in India; Technical Bulletin; ICAR-CSSRI: Karnal, India, 2015; pp. 1–99. [Google Scholar]
- CGWB. Inland Groundwater Salinity in India; Central Ground Water Board, Ministry of Water Resources, Government of India: Faridabad, India, 1997.
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation induced salinity and sodicity hazards on soil and groundwater: An overview of its causes, impacts and mitigation strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Viswanath, S. Sandalwood, an unexplored treasure. Inside Fact Nat. 2014, 1, 10–13. [Google Scholar]
- Rashkow, E.D. Perfumed the axe that laid it low: The endangerment of sandalwood in southern India. Ind. Econ. Soc. Hist Rev. 2014, 51, 41–70. [Google Scholar] [CrossRef]
- Baldovini, N.; Delasalle, C.; Joulain, D. Phytochemistry of the heartwood from fragrant Santalum species: A review. Flavour. Fragr. J. 2011, 26, 7–26. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of sandalwood oil (Santalum album L.). Food Chem. Toxicol. 2008, 46, 421–432. [Google Scholar] [CrossRef]
- Kumar, A.N.A.; Joshi, G.; Ram, H.M. Sandalwood: History, uses, present status and the future. Curr. Sci. 2012, 103, 1408–1416. [Google Scholar]
- Bunney, E.; McInerney, F.A.; Dormontt, E.; Malik, A.; Welti, N.; Wilkins, D.; Plant, M.; Hettiarachchi, D.S.; Thomas, D.; Dowell, A.; et al. Safeguarding sandalwood: A review of current and emerging tools to support sustainable and legal forestry. Plants People Planet 2023, 5, 190–202. [Google Scholar] [CrossRef]
- Mishra, B.; Chakraborty, S.; Viswanath, S. Sandalwood farming in India: Problems and prospects. Prospects. Ind. J. Trop Biodiv. 2018, 26, 1–12. [Google Scholar]
- Nagaveni, H.C.; Vijayalakshmi, G. Growth performance of sandal (Santalum album L.) with different host species. Sandalwood Res. Newsl. 2003, 18, 1–4. [Google Scholar]
- Radomiljac, A.M.; McComb, J.A.; Pate, J.S.; Tennakoon, K.U. Xylem Transfer of Organic Solutes in Santalum album L. (Indian Sandalwood) in Association with Legume and Non-legume Hosts. Ann. Bot. 1998, 82, 675–682. [Google Scholar] [CrossRef]
- Srikantaprasad, D.; Gowda, A.M.; Pushpa, T.N.; Thimmegowda, M.N.; Umesha, K.; Ravikumar, R.L.; Prasanna, K.T. Identification of suitable host for sandalwood cultivation in Northern dry zone of Karnataka. Ind. Crop Prod. 2022, 182, 114874. [Google Scholar] [CrossRef]
- Kubo, M.; Ueda, H.; Park, P.; Kawaguchi, M.; Sugimoto, Y. Reactions of Lotus japonicus ecotypes and mutants to root parasitic plants. J. Plant Physiol. 2009, 166, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Nagaveni, H.C.; Srimathi, R.A. Germinative capacity of floating and sinking sandal seeds. Indian For. 1985, 111, 615–618. [Google Scholar]
- Barrett, D.R.; Fox, J.E.D. Early growth of Santalum album in relation to shade. Aust. J. Bot. 1994, 42, 83–93. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Chittapur, B.M.D.; Lokesh, R. Parasitism ecology of sandalwood (Santalum album L.) for commercial production in the semi-arid tropics. Curr. Sci. 2020, 119, 699–703. [Google Scholar] [CrossRef]
- Nge, F.J.; Ranathunge, K.; Kotula, L.; Cawthray, G.R.; Lambers, H. Strong host specificity of a root hemi-parasite (Santalum acuminatum) limits its local distribution: Beggars can be choosers. Plant Soil 2019, 437, 159–177. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Sharma, S.; Sachan, S.G.; Singh, B.; Chauhan, V.S.; Sayyed, R.Z.; Kaushik, R.; Saxena, A.K. Psychrotrophic Microbes: Biodiversity, mechanisms of adaptation, and biotechnological implications in alleviation of cold stress in plants. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management. Microorganisms for Sustainability; Sayyed, R., Arora, N., Reddy, M., Eds.; Springer: Singapore, 2019; Volume 12, pp. 219–253. [Google Scholar] [CrossRef]
- Ananthapadmanabha, H.S.; Rangaswamy, C.R.; Sarma, C.R.; Nagaveni, H.C.; Jain, S.H.; Venkatesan, K.R.; Krishnappa, H.P. Host requirement of sandal (Santalum album L.). Ind. For. 1984, 110, 264–268. [Google Scholar]
- Azeem, M.; Pirjan, K.; Qasim, M.; Mahmood, A.; Javed, T.; Muhammad, H.; Yang, S.; Dong, R.; Ali, B.; Rahimi, M. Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci. Rep. 2023, 13, 2895. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Singla, R. Nitrate reductase activity in roots and leaves of chickpea cultivars under salt stress. Span. J. Agric. Res. 2005, 3, 248–252. [Google Scholar] [CrossRef]
- Mann, A.; Kumar, A.; Saha, M.; Lata, C.; Kumar, A. Stress induced changes in osmoprotectants, ionic relations, antioxidants activities and protein profiling characterize Sporobolus marginatus Hochst. Ex A. Rich. Salt tolerance mechanism. Ind. J. Expt. Biol. 2019, 57, 672–679. [Google Scholar]
- Rai, S.N. Status and cultivation of sandalwood in India. In Proceedings of the Symposium on Sandalwood in the Pacific, Honolulu, HI, USA, 9–11 April 1990; pp. 66–71. [Google Scholar]
- Pate, J.S. Haustoria in action: Case studies of nitrogen acquisition by woody xylem-tapping hemiparasites from their hosts. Protoplasma 2001, 215, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Sharif, I.; Aleem, S.; Farooq, J.; Rizwan, M.; Younas, A.; Sarwar, G.; Chohan, S.M. Salinity stress in cotton: Effects, mechanism of tolerance and its management strategies. Physiol. Mol. Biol. Plants 2019, 25, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Meriem, B.F.; Kaouther, Z.; Chérif, H.; Tijani, M.; André, B. Effect of priming on growth, biochemical parameters and mineral composition of different cultivars of coriander (Coriandrum sativum L.) under salt stress. J. Stress Physiol. Biochem. 2014, 10, 84–109. [Google Scholar]
- Çelik, Ö.; Atak, Ç. Evaluation of proline accumulation and delta (1)-pyrroline-5-carboxylate synthetase (P5CS) gene expression during salinity stress in two soybean (Glycine max L. Merr.) varieties. Pol. J. Environ. Stud. 2012, 21, 559–564. [Google Scholar]
- Balasubramanian, A.; Prasath, C.H.; Radhakrishnan, S.; Sivaprakash, M. Host-specific influence on early growth and physiological attributes of sandal (Santalum album) grown in farmlands. J. Environ. Biol. 2021, 42, 1162–1167. [Google Scholar] [CrossRef]
- Sheoran, P.; Basak, N.; Kumar, A.; Yadav, R.K.; Singh, R.; Sharma, R.; Kumar, S.; Singh, R.K.; Sharma, P.C. Ameliorants and salt tolerant varieties improve rice wheat production in soils undergoing sodification with alkali water irrigation in Indo-Gangetic Plains of India. Agric. Water Manag. 2021, 243, 106492. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, N.; Kaur, G.; Kumar, A.; Mann, A. Variability of durum wheat genotypes in terms of physio-biochemical traits against salinity stress. Cereal. Res. Comm. 2021, 49, 45–54. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Biol. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Mehrotra, S.; Dimkpa, C.O.; Goyal, V. Survival mechanisms of chickpea (Cicer arietinum) under saline conditions. Plant Physiol. Biochem. 2023, 205, 108168. [Google Scholar] [CrossRef]
- Wang, Y.; Nii, N. Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotechnol. 2000, 75, 623–627. [Google Scholar] [CrossRef]
- Dhansu, P.; Kulshreshtha, N.; Kumar, R.; Raja, A.K.; Pandey, S.K.; Goel, V.; Ram, B. Identification of drought-tolerant co-canes based on physiological traits, yield attributes and drought tolerance indices. Sugar Tech. 2021, 23, 747–761. [Google Scholar] [CrossRef]
- Dhaniklal, G. Influence of Host Plants on Soil Moisture Stress on the Water Relations in Sandal. Master’s Thesis, Kerala Agricultural University, Thrissur, India, 2006. [Google Scholar]
- Světlíková, P.; Hájek, T.; Těšitel, J. Water-stress physiology of Rhinanthus alectorolophus, a root-hemiparasitic plant. PLoS ONE 2018, 13, e0200927. [Google Scholar] [CrossRef]
- Rocha, D.; Ashokan, P.K.; Santhoshkumar, A.V.; Anoop, E.V.; Sureshkumar, P. Influence of host plant on the physiological attributes of field-grown sandal tree (Santalum album). J. Trop For. Sci. 2014, 26, 166–172. Available online: https://www.jstor.org/stable/23723901 (accessed on 5 November 2023).
- Ma, G.H.; He, Y.M.; Zhang, J.F.; Chen, F.L. Studies on semi-parasitic sandalwood seedlings. J. Trop Subtrop. Bot. 2005, 13, 233–238. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, M.K.; Tiwari, S.; Gupta, N.; Tripathi, N.; Mishra, N. Evaluation of soybean (Glycine max L.) genotypes on the basis of biochemical contents and anti-oxidant enzyme activities. Leg Res. 2021, 44, 1419–1429. [Google Scholar] [CrossRef]
- Muche, M.; Muasya, A.M.; Tsegay, B.A. Biology and resource acquisition of mistletoes, and the defense responses of host plants. Ecol. Proc. 2022, 11, 24. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. Afr. J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Gama, P.B.S.; Tanaka, K.; Eneji, A.E.; Eltayeb, A.E.; Siddig, K.E. Salt-induced stress effects on biomass, photosynthetic rate, and reactive oxygen species-scavenging enzyme accumulation in common bean. J. Plant Nutri. 2009, 32, 837–854. [Google Scholar] [CrossRef]
- Qing, D.J.; Lu, H.F.; Li, N.; Dong, H.T.; Dong, D.F.; Li, Y.Z. Comparative profiles of gene expression in leaves and roots of maize seedlings under conditions of salt stress and the removal of salt stress. Plant Cell Physiol. 2009, 50, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant Divers. 2016, 1, 183–189. [Google Scholar] [CrossRef]
- Mahmud, S.; Sharmin, S.; Chowdhury, B.L.; Hossain, M.A. Effect of salinity and alleviating role of methyl jasmonate in some rice varieties. Asian J. Plant Sci. 2017, 16, 87–93. [Google Scholar] [CrossRef]
- Kumar, A.; Mann, A.; Kumar, A.; Kumar, N.; Meena, B.L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Internat. J. Phytorem. 2021, 23, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Azooz, M.M.; Metwally, A.; Abou-Elhamd, M.F. Jasmonate-induced tolerance of Hassawi okra seedlings to salinity in brackish water. Acta Physiol Plant. 2015, 37, 1–13. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Hilge, M. Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger. J. Biol. Chem. 2012, 287, 31641–31649. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Maathuis, F.J. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Villa-Castorena, M.; Ulery, A.L.; Catalán-Valencia, E.A.; Remmenga, M.D. Salinity and nitrogen rate effects on the growth and yield of chile pepper plants. Soil Sci. Soc. Am. J. 2003, 67, 1781–1789. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Pooja, P.; Nandwal, A.S.; Chand, M.; Pal, A.; Kumari, A.; Rani, B.; Goel, V.; Kulshreshtha, N. Soil moisture deficit induced changes in antioxidative defense mechanism of sugarcane (Saccharum officinarum) varieties differing in maturity. Ind. J. Agric. Sci. 2020, 90, 507–512. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.K. Plant salt tolerance. In Plant Responses to Abiotic Stress. Topics in Current Genetics; Hirt, H., Shinozaki, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 4, pp. 241–270. [Google Scholar]
- Rani, B.; Madan, S.; Sharma, K.D.; Pooja Kumar, A. Influence of arbuscular mycorrhiza on antioxidative system of wheat (Triticum aestivum) under drought stress. Ind. J. Agric. Sci. 2018, 88, 289–295. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Polash, M.A.S.; Sakil, M.A.; Hossain, M.A. Plants responses and their physiological and biochemical defense mechanisms against salinity: A review. Trop Plant Res. 2019, 6, 250–274. [Google Scholar] [CrossRef]
- Akbari, A.; Gharanjik, S.; Koobaz, P.; Sadeghi, A. Plant growth promoting Streptomyces strains are selectively interacting with the wheat cultivars especially in saline conditions. Heliyon 2020, 6, e03445. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of non-invasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, A.K.; Singh, K.; Lata, C.; Kumar, A.; Krishnamurthy, S.L.; Kumar, P. Diurnal changes and effect of elevated CO2 on gas exchange under individual and interactive salt and water stress in wheat (Triticum aestivum). Ind. J. Agric. Sci. 2019, 89, 763. [Google Scholar] [CrossRef]
- Kaur, G.; Sanwal, S.K.; Sehrawat, N.; Kumar, A.; Kumar, N.; Mann, A. Getting to the roots of Cicer arietinum L. (chickpea) to study the effect of salinity on morpho-physiological, biochemical and molecular traits. Saudi J. Biol. Sci. 2022, 29, 103464. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Zabeti, M.; Lefferts, L.; Brem, G.; Seshan, K. Conversion of lignocellulosic biomass to green fuel oil over sodium based catalysts. Biores. Technol. 2013, 142, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Foyer, C.H. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Plant 1978, 139, 9–17. [Google Scholar] [CrossRef] [PubMed]
- IRRI. International Rice Research Institute (IRRI), Philippines. 2013. Available online: http://bbi.irri.org/products (accessed on 12 July 2023).
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, UK, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]



| Salinity/ Host Species | Chlorophyll (mg g−1) | Carotenoids (mg g−1 FW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | |
| M. dubia | 1.26 AB ± 0.09 | 1.19 A ± 0.10 | 1.09 BC ± 0.04 | 0.94 A ± 0.03 | 1.12 b | 1.69 A ± 0.18 | 2.17 A ± 0.23 | 2.48 AB ± 0.18 | 3.42 AB ± 0.21 | 2.44 a |
| C. equisetifolia | 1.41 A ± 0.10 | 1.20 A ± 0.02 | 1.07 C ± 0.02 | 0.79 B ± 0.03 | 1.12 b | 1.54 A ± 0.17 | 1.84 A ± 0.18 | 2.04 CD ± 0.19 | 2.63 CD ± 0.18 | 2.01 cd |
| C. aurantium | 1.36 AB ± 0.03 | 1.24 A ± 0.03 | 1.18 A ± 0.03 | 0.96 A ± 0.04 | 1.18 a | 1.80 A ± 0.09 | 2.19 A ± 0.16 | 2.43 AB ± 0.17 | 2.98 ABCD ± 0.19 | 2.35 ab |
| P. emblica | 1.40 A ± 0.05 | 1.21 A ± 0.04 | 1.15 AB ± 0.03 | 1.01 A ± 0.04 | 1.19 a | 1.48 A ± 0.33 | 1.82 AB ± 0.13 | 1.99 CD ± 0.26 | 2.37 D ± 0.22 | 1.91 d |
| S. cumini | 1.22 B ± 0.04 | 1.02 B ± 0.04 | 0.98 D ± 0.03 | 0.84 B ± 0.02 | 1.01 c | 1.50 A ± 0.21 | 1.85 A ± 0.08 | 2.01 CD ± 0.10 | 2.49 CD ± 0.10 | 1.96 cd |
| A. ampliceps | 0.87 C ± 0.04 | 0.73 C ± 0.03 | 0.72 E ± 0.03 | 0.59 C ± 0.04 | 0.73 d | 1.77 A ± 0.22 | 2.19 A ± 0.20 | 2.54 A ± 0.26 | 2.90 BCD ± 0.13 | 2.35 ab |
| P. granatum | 0.42 E ± 0.01 | 0.40 F ± 0.04 | 0.34 H ± 0.03 | 0.30 E ± 0.01 | 0.36 h | 1.04 B ± 0.19 | 1.25 B ± 0.14 | 1.29 E ± 0.21 | 1.50 E ± 0.20 | 1.27 e |
| A. indica | 0.71 D ± 0.03 | 0.62 CD ± 0.04 | 0.59 F ± 0.02 | 0.40 D ± 0.02 | 0.58 e | 1.52 A ± 0.20 | 1.88 A ± 0.34 | 2.25 BC ± 0.30 | 3.02 ABC ± 0.30 | 2.17 bc |
| L. leucocephala | 0.61 D ± 0.05 | 0.46 EF ± 0.03 | 0.38 H ± 0.03 | 0.32 E ± 0.01 | 0.44 g | 1.48 A ± 0.05 | 1.75 AB ± 0.14 | 1.94 D ± 0.17 | 2.40 CD ± 0.18 | 1.89 d |
| D. sissoo | 0.65 D ± 0.06 | 0.55 DE ± 0.02 | 0.51 G ± 0.02 | 0.46 D ± 0.03 | 0.54 f | 1.69 A ± 0.19 | 2.12 A ± 0.19 | 2.47 AB ± 0.22 | 3.60 A ± 0.29 | 2.47 a |
| Mean | 0.99 a | 0.86 b | 0.80 c | 0.66 d | 1.55 d | 1.91 c | 2.14 b | 2.73 a | ||
| HSD0.05(Host) | 0.16 | 0.13 | 0.07 | 0.08 | 0.03 | 0.34 | 0.59 | 0.28 | 0.62 | 0.22 |
| HSD0.05(Salinity) | 0.04 | 0.32 | ||||||||
| HSD0.05(Host × Salinity) | 0.07 | 0.54 | ||||||||
| Salinity/ Host Species | Photosynthetic Rate (µ mol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | |
| M. dubia | 4.84 B ± 0.26 | 4.07 B ± 0.10 | 3.70 B ± 0.30 | 2.92 B ± 0.31 | 3.88 c | 1.39 E ± 0.15 | 1.21 D ± 0.12 | 1.07 C ± 0.10 | 0.96 C ± 0.15 | 1.16 g |
| C. equisetifolia | 3.53 C ± 0.12 | 2.75 DE ± 0.13 | 1.98 D ± 0.11 | 1.43 DE ± 0.10 | 2.42 g | 1.24 E ± 0.14 | 1.07 D ± 0.11 | 0.77 C ± 0.07 | 0.61 E ± 0.08 | 0.92 h |
| C. aurantium | 2.59 DE ± 0.05 | 1.57 F ± 0.14 | 1.17 EF ± 0.14 | 0.23 F ± 0.08 | 1.39 i | 2.27 D ± 0.12 | 1.43 D ± 0.17 | 1.00 C ± 0.09 | 0.53 E ± 0.05 | 1.31 g |
| P. emblica | 3.09 CDE ± 0.07 | 2.48 E ± 0.11 | 1.72 DE ± 0.08 | 1.05 E ± 0.18 | 2.08 h | 2.23 D ± 0.15 | 1.38 D ± 0.13 | 1.05 C ± 0.10 | 0.62 DE ± 0.10 | 1.32 g |
| S. cumini | 2.47 E ± 0.19 | 1.08 F ± 0.08 | 0.64 F ± 0.08 | 0.25 F ± 0.05 | 1.11 j | 3.33 C ± 0.17 | 2.90 C ± 0.19 | 1.15 C ± 0.13 | 0.58 E ± 0.07 | 1.99 f |
| A. ampliceps | 4.68 B ± 0.32 | 3.66 BC ± 0.29 | 2.40 CD ± 0.13 | 0.95 E ± 0.15 | 2.92 e | 6.32 A ± 0.13 | 4.88 A ± 0.16 | 3.19 A ± 0.26 | 1.87 B ± 0.11 | 4.06 b |
| P. granatum | 6.47 A ± 0.65 | 3.20 CD ± 0.13 | 2.84 C ± 0.11 | 2.01 C ± 0.17 | 3.63 d | 4.51 B ± 0.37 | 3.03 C ± 0.10 | 2.27 B ± 0.15 | 1.66 B ± 0.12 | 2.87 d |
| A. indica | 3.42 ± 0.10 | 2.92 DE ± 0.11 | 2.29 CD ± 0.07 | 1.90 CD ± 0.14 | 2.63 f | 3.40 C ± 0.12 | 2.84 C ± 0.20 | 2.21 B ± 0.14 | 0.93 CD ± 0.05 | 2.34 e |
| L. leucocephala | 7.18 A ± 0.49 | 5.20 A ± 0.24 | 3.85 B ± 0.59 | 2.62 B ± 0.32 | 4.71 b | 6.69 A ± 0.11 | 4.93 A ± 0.30 | 3.38 A ± 0.20 | 1.97 B ± 0.15 | 4.24 a |
| D. sissoo | 6.34 A ± 0.12 | 5.48 A ± 0.43 | 4.83 A ± 0.12 | 3.86 A ± 0.12 | 5.12 a | 4.60 B ± 0.22 | 3.88 B ± 0.11 | 3.29 A ± 0.12 | 2.55 A ± 0.13 | 3.58 c |
| Mean | 4.46 a | 3.24 b | 2.54 c | 1.72 d | 3.60 a | 2.76 b | 1.94 c | 1.23 d | ||
| HSD0.05(Host) | 0.845 | 0.615 | 0.700 | 0.541 | 0.07 | 0.54 | 0.48 | 0.39 | 0.31 | 0.16 |
| HSD0.05(Salinity) | 0.14 | 0.12 | ||||||||
| HSD0.05(Host × Salinity) | 0.28 | 0.41 | ||||||||
| Alinity/ Host Species | K+/Na+ | Ca2+/Na+ | Ca2+/Mg2+ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | Control | ECiw 3.0 dS m−1 | ECiw 6.0 dS m−1 | ECiw 9.0 dS m−1 | Mean | |
| M. dubia | 2.17 A ± 0.09 | 1.72 A ± 0.03 | 1.29 AB ± 0.02 | 0.89 B ± 0.02 | 1.52 a | 1.47 A ± 0.062 | 1.15 A ± 0.007 | 0.86 AB ± 0.008 | 0.66 B ± 0.019 | 1.04 a | 1.95 ABC ± 0.03 | 1.87 AB ± 0.12 | 1.80 A ± 0.02 | 1.75 AB ± 0.11 | 1.84 bc |
| C. equisetifolia | 1.88 BCD ± 0.07 | 1.43 BC ± 0.06 | 1.09 C ± 0.06 | 0.72 CD ± 0.04 | 1.28 c | 1.28 B ± 0.009 | 0.96 B ± 0.069 | 0.73 C ± 0.009 | 0.51 D ± 0.009 | 0.87 c | 2.12 AB ± 0.09 | 1.99 A ± 0.12 | 1.85 A ± 0.06 | 1.72 AB ± 0.05 | 1.92 ab |
| C. aurantium | 1.79 CD ± 0.06 | 1.37 BCD ± 0.02 | 1.01 CD ± 0.05 | 0.64 E ± 0.01 | 1.20 e | 1.09 CD ± 0.030 | 0.79 CD ± 0.001 | 0.60 DE ± 0.017 | 0.40 EF ± 0.012 | 0.72 d | 1.99 ABC ± 0.04 | 1.86 AB ± 0.01 | 1.72 AB ± 0.08 | 1.60 BC ± 0.08 | 1.79 c |
| P. emblica | 1.82 CD ± 0.01 | 1.38 BCD ± 0.07 | 1.00 CD ± 0.02 | 0.65 E ± 0.03 | 1.21 de | 0.98 DE ± 0.036 | 0.71 D ± 0.034 | 0.53 EF ± 0.031 | 0.36 G ± 0.014 | 0.64 ef | 2.05 AB ± 0.13 | 1.95 A ± 0.09 | 1.78 A ± 0.05 | 1.66 AB ± 0.06 | 1.86 abc |
| S. cumini | 1.67 D ± 0.13 | 1.22 D ± 0.08 | 0.91 DE ± 0.01 | 0.63 E ± 0.01 | 1.11 f | 0.99 DE ± 0.055 | 0.72 D ± 0.044 | 0.51 F ± 0.010 | 0.37 FG ± 0.015 | 0.65 ef | 2.00 ABC ± 0.06 | 1.89 A ± 0.06 | 1.73 AB ± 0.08 | 1.59 BC ± 0.03 | 1.80 c |
| A. ampliceps | 1.90 BC ± 0.04 | 1.46 B ± 0.03 | 1.03 C ± 0.03 | 0.76 C ± 0.02 | 1.29 c | 0.94 E ± 0.037 | 0.69 D ± 0.034 | 0.48 F ± 0.009 | 0.35 G ± 0.018 | 0.62 f | 1.79 C ± 0.06 | 1.67 B ± 0.09 | 1.54 B ± 0.05 | 1.44 C ± 0.02 | 1.61 d |
| P. granatum | 1.76 CD ± 0.08 | 1.29 CD ± 0.03 | 0.87 E ± 0.03 | 0.66 DE ± 0.03 | 1.14 ef | 1.04 CDE ± 0.054 | 0.76 CD ± 0.035 | 0.53 EF ± 0.023 | 0.38 FG ± 0.020 | 0.68 e | 2.01 ABC ± 0.09 | 1.88 A ± 0.06 | 1.75 AB ± 0.03 | 1.62 BC ± 0.02 | 1.81 c |
| A. indica | 2.10 AB ± 0.10 | 1.67 A ± 0.07 | 1.23 B ± 0.03 | 0.86 B ± 0.01 | 1.47 b | 1.36 AB ± 0.069 | 1.05 AB ± 0.019 | 0.79 BC ± 0.045 | 0.57 C ± 0.002 | 0.94 b | 2.07 AB ± 0.04 | 1.98 A ± 0.08 | 1.91 A ± 0.10 | 1.81 A ± 0.02 | 1.94 |
| L. leucocephala | 1.86 CD ± 0.01 | 1.39 BC ± 0.04 | 1.10 C ± 0.05 | 0.73 C ± 0.02 | 1.27 cd | 1.13 C ± 0.011 | 0.82 C ± 0.010 | 0.63 D ± 0.017 | 0.43 E ± 0.005 | 0.75 d | 2.13 A ± 0.10 | 1.98 A ± 0.01 | 1.84 A ± 0.07 | 1.71 AB ± 0.08 | 1.92 a |
| D. sissoo | 2.13 A ± 0.10 | 1.73 A ± 0.07 | 1.39 A ± 0.04 | 0.98 A ± 0.04 | 1.56 a | 1.37 AB ± 0.039 | 1.12 A ± 0.044 | 0.91 A ± 0.042 | 0.71 A ± 0.013 | 1.03 a | 1.90 BC ± 0.06 BC | 1.84 AB ± 0.06 | 1.78 A ± 0.12 | 1.69 AB ± 0.03 | 1.81 c |
| Mean | 1.91 a | 1.47 b | 1.09 c | 0.75 d | 1.17 a | 0.88 b | 0.66 c | 0.47 d | 2.00 a | 1.89 b | 1.77 c | 1.66 d | |||
| HSD0.05(Host) | 0.22 | 0.16 | 0.11 | 0.07 | 0.07 | 0.12 | 0.10 | 0.07 | 0.04 | 0.04 | 0.23 | 0.21 | 0.21 | 0.18 | 0.09 |
| HSD0.05(Salinity) | 0.06 | 0.03 | 0.07 | ||||||||||||
| HSD0.05(Host × Salinity) | 0.17 | 0.10 | 0.24 | ||||||||||||
| Environments | Linear Models for Predicted Biomass | Coefficient of Determination (R2) |
|---|---|---|
| Control | ~74.29 + 0.82 RWC + (−8.05)Pn + (−3.62) K + (−40.57) Na/K + (−90.35) MDA + 64.27APX + (−0.32) POX + 3.76 SOD + 7.75 GR + 8.48 WP + 17.57 Dia | 0.98 |
| Low salinity stress (ECiw~3 dS m−1) | ~45.890 + (−8.48) Pn+ (−15.040) Chl + (−1.530) MI + (−2.920) SPAD + 26.070 Na + 13.810 K + (−81.030) MDA + 46.070 APX + 0.730 SOD + 10.710 Dia | 0.98 |
| Moderate salinity stress (ECiw~6 dS m−1) | ~−26.550 +−5.42 Pn+ (−0.35) MI + (−1.83) SPAD + 6.63 Na + 8.84 K + 0.11 POX + 30.59 GR + 3.05 WP + 7.83 Dia + (−0.31) RWC | 0.99 |
| Higher salinity stress (ECiw~9dS m−1) | ~−66.140 + 0.440 RWC + (−3.080) E + (−9.290) Chl + (−0.780) MI + (−1.510) SPAD + 3.380 Na + 5.660 K + 27.080 MDA + (−11.110) APX + 62.380 CAT + 15.860 GR + 13.150 Dia | 0.99 |
| Environments | |||
|---|---|---|---|
| Control (Good Quality Irrigation Water) | Low Salinity Stress (ECiw~3 dS m−1) | Moderate Salinity Stress (ECiw~6 dS m−1) | Higher Salinity Stress (ECiw~9 dS m−1) |
| M. dubia (1) | M. dubia (1) | D. sissoo (1) | D. sissoo (1) |
| D. sissoo (2) | D. sissoo (2) | M. dubia (2) | M. dubia (2) |
| C. equisetifolia (3) | A. indica (3) | A. indica (3) | A. indica (3) |
| A. indica (4) | C. equisetifolia (4) | C. equisetifolia (4) | C. equisetifolia (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, K.; Kumar, A.; Kumar, R.; Kumar, N.; Kumar, A.; Bhardwaj, A.K.; Verma, R.C.; Sharma, P. Host Plant Modulated Physio-Biochemical Process Enhances Adaptive Response of Sandalwood (Santalum album L.) under Salinity Stress. Plants 2024, 13, 1162. https://doi.org/10.3390/plants13081162
Verma K, Kumar A, Kumar R, Kumar N, Kumar A, Bhardwaj AK, Verma RC, Sharma P. Host Plant Modulated Physio-Biochemical Process Enhances Adaptive Response of Sandalwood (Santalum album L.) under Salinity Stress. Plants. 2024; 13(8):1162. https://doi.org/10.3390/plants13081162
Chicago/Turabian StyleVerma, Kamlesh, Ashwani Kumar, Raj Kumar, Naresh Kumar, Arvind Kumar, Ajay Kumar Bhardwaj, Ramesh Chander Verma, and Prashant Sharma. 2024. "Host Plant Modulated Physio-Biochemical Process Enhances Adaptive Response of Sandalwood (Santalum album L.) under Salinity Stress" Plants 13, no. 8: 1162. https://doi.org/10.3390/plants13081162
APA StyleVerma, K., Kumar, A., Kumar, R., Kumar, N., Kumar, A., Bhardwaj, A. K., Verma, R. C., & Sharma, P. (2024). Host Plant Modulated Physio-Biochemical Process Enhances Adaptive Response of Sandalwood (Santalum album L.) under Salinity Stress. Plants, 13(8), 1162. https://doi.org/10.3390/plants13081162