Decrease in Available Soil Water Storage Capacity Reduces Vitality of Young Understorey European Beeches (Fagus sylvatica L.)—A Case Study from the Black Forest, Germany
Abstract
:1. Introduction
2. Results
2.1. Available Soil Water Storage Capacity (ASWSC) in Stand
| Model parameters | Model estimates | Standard error | t | p value |
|---|---|---|---|---|
| Model constant | −32.390 | 67.257 | −0.482 | 0.636 |
| Dependent variable: ASWSC | ||||
| Independent variables | ||||
| Soil depth up to bedrock | 1.568 | 0.126 | 12.432 | 0.000 |
| Slope of soil profiles | 0.350 | 0.418 | 0.836 | 0.415 |
| Sand | 0.640 | 0.704 | 0.908 | 0.376 |
| Clay | 0.120 | 0.637 | 0.188 | 0.853 |
| Silt | 0.170 | 0.703 | 0.241 | 0.812 |
| Soil skeleton content | −0.834 | 0.099 | −8.421 | 0.000 |
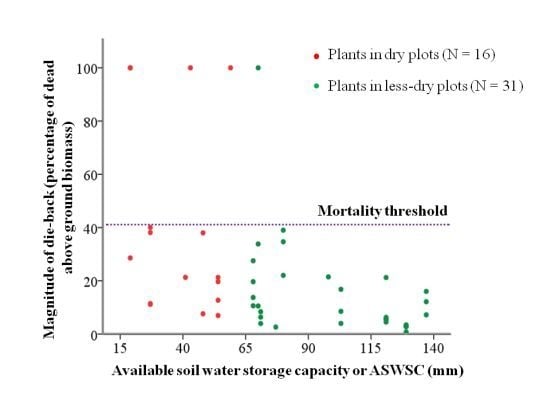
2.2. Relation between ASWSC and Crown Dieback



| Parameters | Model estimate (β) | Standard error for model estimate | Wald Chi square | df | p value |
|---|---|---|---|---|---|
| Less dry plots vs. Dry plots | −2.303 | 1.169 | 3.879 | 1 | 0.0389 |
| Model constant | 3.401 | 1.017 | 11.195 | 1 | 0.0008 |
2.3. Partial Dieback in Different Crown Compartments

2.4. Summer Drought of 2003 and Basal Area Increment



3. Discussion
4. Experimental
4.1. Study Site and Sampling Design

4.2. Collection of Morphological Data
4.3. Collection of Soil Data and Quantification of ASWSC
4.3.1. Collection of Soil Data from Forest Stand
4.3.2. Quantification of ASWSC
| Dry plots | Less dry plots | ||||
|---|---|---|---|---|---|
| Serial no. | Plot no. | Plot ASWSC (mm) | Serial no. | Plot no. | Plot ASWSC (mm) |
| 1 | 1/1 | 22 | 1 | 1/5 | 71 |
| 2 | 1/2 | 21 | 2 | 1/6 | 68 |
| 3 | 1/3 | 41 | 3 | 2/3 | 98 |
| 4 | 1/4 | 59 | 4 | 2/6 | 70 |
| 5 | 2/1 | 52 | 5 | 3/1 | 129 |
| 6 | 2/2 | 19 | 6 | 3/2 | 103 |
| 7 | 2/4 | 27 | 7 | 3/3 | 77 |
| 8 | 2/5 | 43 | 8 | 3/5 | 80 |
| 9 | 3/4 | 54 | 9 | 4/1 | 103 |
| 10 | 3/6 | 54 | 10 | 4/2 | 121 |
| 11 | 4/3 | 27 | 11 | 4/4 | 137 |
| 12 | 4/6 | 48 | 12 | 4/5 | 93 |
| average ASWSC 39 | average ASWSC 96 | ||||
4.4. Quantification of Crown Dieback
4.4.1. Development of Allometric Equations for above Ground Biomass from Harvested Plants

| Statistical tests | Dry plots | Less dry plots | Dead tree |
|---|---|---|---|
| t-test | t(39) = 0.72 p > 0.05 | t(39) = 1.24 p > 0.05 | t(41) = −0.22 p > 0.05 |

4.4.2. Simulation of Dead above Ground Biomass
4.4.3. Calculation of the Proportion Dead above Ground Biomass (Quantitative Estimation of Crown-Dieback)

4.5. Tree-Ring Analysis for Calculation of Basal Area Increment (BAI) for the Growth Study

4.6. Statistical Analyses
4.6.1. First Hypothesis
4.6.2. Second Hypothesis
4.6.3. Third Hypothesis
4.6.4. Factors Influencing ASWSC
5. Conclusions
Supplementary Files
Supplementary File 1Supplementary File 2Acknowledgments
Contributions of Co-Authors
Conflicts of Interest
References
- IPCC, Climate Change 2007: The Physical Science Basis; Intergovernmental Panel on Climate Change: New York, NY, USA, 2007; p. 996.
- Mayer, H.; Holst, T.; Brugger, U.; Kirchassner, A. Trends of the forest significant climate variables air temperature and precipitation in south-west Germany from 1950 to 2000. Allgemeine Forst- und Jagd-Zeitung 2005, 176, 45–56. [Google Scholar]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogee, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Berry, J.A.; Field, C.B. Linking definitions, mechanisms, and modeling of drought-induced tree death. Trends Plant Sci. 2012, 17, 693–700. [Google Scholar] [CrossRef]
- Kohler, M.; Kockemann, B.; Peichl, M.; Schmitt, J.; Reif, A. Impacts of the drought 2003 on the crown condition of suppressed and intermediate beech trees (Fagus sylvatica L.) at the ecotone between beech and downy oak forest in the nature reserve Innerberg, Sudbaden. Allgemeine Forst- und Jagd-Zeitung 2006, 177, 86–90. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Bowman, D.; Nichols, S.; Delzon, S.; Burlett, R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 2010, 188, 533–542. [Google Scholar] [CrossRef]
- Coll, L.; Balandier, P.; Picon-Cochard, C.; Prevosto, B.; Curt, T. Competition for water between beech seedlings and surrounding vegetation in different light and vegetation composition conditions. Ann. For. Sci. 2003, 60, 593–600. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Breda, N.; Ulrich, E.; Granier, A. Climate-tree-growth relationships of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees Struct. Funct. 2005, 19, 385–401. [Google Scholar] [CrossRef]
- Robson, T.M.; Rodriguez-Calcerrada, J.; Sanchez-Gomez, D.; Aranda, I. Summer drought impedes beech seedling performance more in a sub-Mediterranean forest understory than in small gaps. Tree Physiol. 2009, 29, 249–259. [Google Scholar]
- Van Hees, A.F.M. Growth and morphology of pedunculate oak (Quercus robur L.) and beech (Fagus sylvatica L.) seedlings in relation to shading and drought. Ann. Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Gartner, S.; Reif, A.; Xystrakis, F.; Sayer, U.; Bendagha, N.; Matzarakis, A. The drought tolerance limit of Fagus sylvatica forest on limestone in southwestern Germany. J. Veg. Sci. 2008, 19, 757–768. [Google Scholar]
- Topoliantz, S.; Ponge, J.F. Influence of site conditions on the survival of Fagus sylvatica seedlings in an old-growth beech forest. J. Veg. Sci. 2000, 11, 369–374. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Seletkovic, I.; Potocic, N.; Ugarkovic, D.; Jazbec, A.; Pernar, R.; Seletkovic, A.; Benko, M. Climate and relief properties influence crown condition of common beech (Fagus sylvatica L.) on the Medvednica massif. Period. Biol. 2009, 111, 435–441. [Google Scholar]
- Czajkowski, T.; Kuhling, M.; Bolte, A. Impact of the 2003 summer drought on growth of beech sapling natural regeneration (Fagus sylvatica L.) in north-eastern Central Europe. Allgemeine Forst- und Jagd-Zeitung 2005, 176, 133–143. [Google Scholar]
- Rood, S.B.; Patino, S.; Coombs, K.; Tyree, M.T. Branch sacrifice: Cavitation-associated drought adaptation of riparian cottonwoods. TreesStruct. Funct. 2000, 14, 248–257. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L. Hydraulic and carbohydrate changes in experimental drought-induced mortality of saplings in two conifer species. Tree Physiol. 2013, 33, 252–260. [Google Scholar] [CrossRef]
- Puettmann, K.; Coates, K.D.; Messier, C. A Critique of Silviculture: Managing for Complexity; Island Press: Washington, DC, USA, 2009; p. 189. [Google Scholar]
- Pedersen, B.S. The role of stress in the mortality of midwestern oaks as indicated by growth prior to death. Ecology 1998, 79, 79–93. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Larcher, W. Plant Ecophysiology; Ulmer Verlag: Stuttgart, Germany, 2001. [Google Scholar]
- Manion, P.D. Tree Disease Concepts; Prentice Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Cochard, H.; Lemoine, D.; Dreyer, E. The effects of acclimation to sunlight on the xylem vulnerability to embolism in Fagus sylvatica L. Plant Cell Environ. 1999, 22, 101–108. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Rust, S.; Roloff, A. Acclimation of crown structure to drought in Quercus robur L.—intra- and inter-annual variation of abscission and traits of shed twigs. Basic Appl. Ecol. 2004, 5, 283–291. [Google Scholar]
- Schulze, E.-D.; Beck, E.; Muller-Hohenstein, K. Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2005; p. 702. [Google Scholar]
- Evenari, M.; Shanan, L.; Tadmor, N. The Negev: The Challenge of a Desert; Harvard University Press: Cambridge, MA, USA, 1982; p. 437. [Google Scholar]
- Rust, S.; Roloff, A. Reduced photosynthesis in old oak (Quercus robur): The impact of crown and hydraulic architecture. Tree Physiol. 2002, 22, 597–601. [Google Scholar] [CrossRef]
- Eckstein, D.; Richter, K.; Aniol, R.W.; Quiehl, F. Dendroclimatological investigations of the beech decline in the southwestern part of the Vogelsberg (Hesse, West-Germany). Forstwissenschaftliches Centralblatt 1984, 103, 274–290. [Google Scholar] [CrossRef]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought responses at leaf, stem and fine root levels of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees in dry and wet years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Brubaker, L.B. Responses of tree populations to climatic-change. Vegetatio 1986, 67, 119–130. [Google Scholar] [CrossRef]
- Von Lüpke, B. Silvicultural methods of oak regeneration with special respect to shade tolerant mixed species. For. Ecol. Manag. 1998, 106, 19–26. [Google Scholar] [CrossRef]
- Chakraborty, T. Effect of Soil Drought on Vitality and Growth on Juvenile and Understorey Beech (Fagus sylvatica L.) Trees: Case Study from a Rocky Gneiss Outcrop near Freiburg, Black Forest, Germany. Master Thesis; Albert-Ludwigs-University of Freiburg: Freiburg, Germany, 2010. Available online: http://www.freidok.uni-freiburg.de/volltexte/8066/ (accessed on 17 October 2013).
- FVA, Aufnahmeanweisung und Verfahrensbeschreibung permanente Betriebsinventur pBle + pBlf; (Guidebook for Doing Forest Inventory, in German); Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg: Freiburg, Germany, 2004.
- Hodgson, J.M. Soil Survey Field Handbook; Soil Survey of England and Wales, Rothamsted Experimental Station: Harpenden, UK, 1974; Volume 5. [Google Scholar]
- Munsell, A.H. Munsell Soil Color Charts, Revised ed.; MacBeth Divisionof Kollmorgen Instruments Corp: New Windsor, NY, USA, 1994. [Google Scholar]
- Sayer, U. Die Ökologie der Flaumeiche (Quercus pubescens Willd.) und ihrer Hybriden auf Kalkstandorten an ihrer nördlichen Arealgrenze (Untersuchungen zu Boden, Klima und Vegetation); Cramer: Stuttgart, Germany, 2000; Volume 340, p. 198. [Google Scholar]
- Samaras, D. The Vegetation of Greek Fir (Abies cephalonica Loudon) Forests on the Oxia—North Vardousia Mountain System, Central Greece, in Relation to Drought. Ph.D. Thesis; Albert Ludwigs University of Freiburg: Freiburg, Germany, 2012. Available online: http://www.freidok.uni-freiburg.de/volltexte/8642/ (accessed on 17 October 2013).
- FAO, Guidelines for Soil Description; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006.
- Arbeitskreis Standortskartierung, Forstliche Standortsaufnahme, 6th ed.; IHW-Verlag und Verlagsbuchhandlung: Munich, Germany, 2003.
- AG Boden, Bodenkundliche Kartieranleitung: Arbeitsgruppe Boden, 4th ed.; Schweizerbart Verlag: Stuttgart, Germany, 1994.
- Schack-Kirchner, H. Ein Fuzzy-Schlüssel für die Texturschätzung mit der Fingerprobe; Fakultät der Albert-Ludwigs-Universität und Forstliche Versuchs- und Forschungsanstalt, Freiburger Forstliche Forschung, Baden-Württemberg: Freiburg, Germany, 2001. [Google Scholar]
- Schlichting, E.; Blume, H.-P.; Stahr, K. Bodenkundliches Praktikum. Eine Einführung in pedologisches Arbeiten für Ökologen, insbesondere Land- und Forstwirte und für Geowissenschaftler, 2nd ed.; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1995. [Google Scholar]
- Zianis, D.; Muukkonen, P.; Makipaa, R.; Mencuccini, M. Biomass and stem volume equations for tree species in Europe. Silva Fenn. Monogr. 2005, 4, 5–63. [Google Scholar]
- WinDENDRO: Tree Ring, Stem, Wood Density Analysis and Measurement; Regent Inc.: Quebec City, QC, Canada, 2009.
- Leblanc, D.C. Relationships between breast-height and whole-stem growth indexes for red spruce on Whiteface Mountain, New-York. Can. J. For. Res. 1990, 20, 1399–1407. [Google Scholar] [CrossRef]
- Rebetez, M.; Mayer, H.; Dupont, O.; Schindler, D.; Gartner, K.; Kropp, J.P.; Menzel, A. Heat and drought 2003 in Europe: A climate synthesis. Ann. For. Sci. 2006, 63, 569–577. [Google Scholar] [CrossRef]
- IBM Corporation, IBM SPSS Advanced Statistics 20; IBM Corporation: New York, USA, 2011.
- Rich, P.M.; Breshears, D.D.; White, A.B. Phenology of mixed woody-herbaceous ecosystems following extreme events: Net and differential responses. Ecology 2008, 89, 342–352. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar]
- Suarez, M.L.; Ghermandi, L.; Kitzberger, T. Factors predisposing episodic drought-induced tree mortality in Nothofagus—Site, climatic sensitivity and growth trends. J. Ecol. 2004, 92, 954–966. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chakraborty, T.; Saha, S.; Reif, A. Decrease in Available Soil Water Storage Capacity Reduces Vitality of Young Understorey European Beeches (Fagus sylvatica L.)—A Case Study from the Black Forest, Germany. Plants 2013, 2, 676-698. https://doi.org/10.3390/plants2040676
Chakraborty T, Saha S, Reif A. Decrease in Available Soil Water Storage Capacity Reduces Vitality of Young Understorey European Beeches (Fagus sylvatica L.)—A Case Study from the Black Forest, Germany. Plants. 2013; 2(4):676-698. https://doi.org/10.3390/plants2040676
Chicago/Turabian StyleChakraborty, Tamalika, Somidh Saha, and Albert Reif. 2013. "Decrease in Available Soil Water Storage Capacity Reduces Vitality of Young Understorey European Beeches (Fagus sylvatica L.)—A Case Study from the Black Forest, Germany" Plants 2, no. 4: 676-698. https://doi.org/10.3390/plants2040676
APA StyleChakraborty, T., Saha, S., & Reif, A. (2013). Decrease in Available Soil Water Storage Capacity Reduces Vitality of Young Understorey European Beeches (Fagus sylvatica L.)—A Case Study from the Black Forest, Germany. Plants, 2(4), 676-698. https://doi.org/10.3390/plants2040676