Silicon and Nitrate Differentially Modulate the Symbiotic Performances of Healthy and Virus-Infected Bradyrhizobium-nodulated Cowpea (Vigna unguiculata), Yardlong Bean (V. unguiculata subsp. sesquipedalis) and Mung Bean (V. radiata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Cultural Practices and Experimental Design
2.2. Yield Parameters
2.3. Statistical Analysis
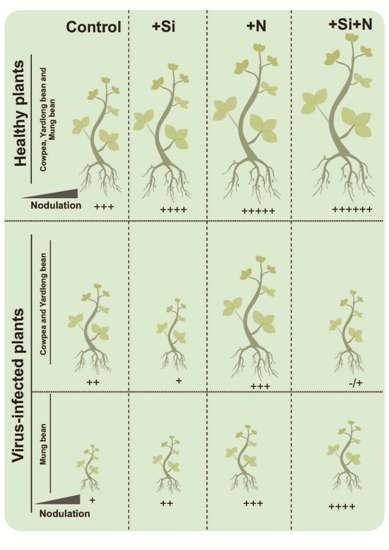
3. Results
3.1. Symptoms and Effects of Virus Infection
3.2. Physiology of Healthy Cowpea
3.3. Physiology of CCMV-Infected Cowpea
3.4. Physiology of CMMV-Infected Cowpea
3.5. Physiology of Healthy and Virus-Infected Yardlong Bean
3.6. Physiology of Healthy Mungbean
3.7. Physiology of CCMV- or CMMV-Infected Mung Bean
4. Discussion
4.1. The Case of Healthy Plants
4.2. The Case of Virus-Infected Plants
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | α-amino acids |
| CCMV | Cowpea chlorotic mottle bromovirus |
| CMMV | Cowpea mild mottle carlavirus |
| Si | silicic acid treatment |
| N | nitrate treatment |
| Si + N | simultaneous addition of silicic acid and nitrate |
References
- Iriti, M.; Varoni, E. Pulses, healthy, and sustainable food sources for feeding the planet. Int. J. Mol. Sci. 2017, 18, 255. [Google Scholar] [CrossRef] [PubMed]
- Gerrano, A.S.; Sternberg, W.; Van Rensburg, J.; Adebola, P.O. Nutritional composition of immature pods in selected cowpea [Vigna unguiculata (L.) Walp.] genotypes in South Africa. Aust. J. Crop Sci. 2017, 11, 134–141. [Google Scholar] [CrossRef]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A legume crop for a challenging environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Oo, A.Z. Genotypic difference in salinity tolerance during early vegetative growth of cowpea (Vigna unguiculata L. Walp.) from Myanmar. Biocatal. Agric. Biotechnol. 2015, 4, 449–455. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Somta, P.; Tomooka, N.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping of pod tenderness and total soluble solid in yardlong bean [Vigna unguiculata (L.) Walp. subsp. unguiculata cv.-gr. sesquipedalis]. Euphytica 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, S.S.; Tripura, P.; Jatav, H.S. Use of phosphorus for maximization of mungbean (Vigna radiata L.) (Wilszeck) productivity under semi-arid condition of Rajasthan, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 612–617. [Google Scholar] [CrossRef]
- Singh, M.; Deokaran, M.J.S.; Bhatt, B.P. Effect of integrated nutrient management on production potential and quality of summer mungbean (Vigna radiata L.). J. Krishi Vigyan 2017, 5, 39–45. [Google Scholar] [CrossRef]
- Tampakaki, A.P.; Fotiadis, C.T.; Ntatsi, G.; Savvas, D. Phylogenetic multilocus sequence analysis of indigenous slow-growing rhizobia nodulating cowpea (Vigna unguiculata L.) in Greece. Syst. Appl. Microbiol. 2017, 40, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Muñoz, N.; Lascano, H.R.; Izaguirre-Mayoral, M.L. The seed-borne Southern bean mosaic virus hinders the early events of nodulation and growth in Rhizobium-inoculated Phaseolus vulgaris L. Funct. Plant Biol. 2017, 44, 208–218. [Google Scholar] [CrossRef]
- Sprent, J.I.; Ardley, J.; James, E.K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 2017, 215, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, F.; Wall, L.; Fabra, A. Starting points in plant-bacteria nitrogen-fixing symbioses: Intercellular invasion of the roots. J. Exp. Bot. 2016, 68, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; da Silva, J.A.T.; Izaguirre-Mayoral, M. Early signaling, synthesis, transport and metabolism of ureides. J. Plant Physiol. 2016, 193, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, Z.; Simon-Sarkadi, L.; Badics, E.; Novák, A.; Mednyánszky, Z.; Szalai, G.; Galiba, G.; Kocsy, G. Redox regulation of free amino acid levels in Arabidopsis thaliana. Physiol. Plant. 2017, 159, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.V.; Veliz, C.G.; Roberts, I.N.; Caputo, C. Phloem transport of amino acids is differentially altered by phosphorus deficiency according to the nitrogen availability in young barley plants. Plant Growth Regul. 2017, 82, 151–160. [Google Scholar] [CrossRef]
- Izaguirre-Mayoral, M.L.; Garrido, M.J. Propyl gallate, a free radical scavenger, counteracts the benefits of exogenously applied salicylic acid and aggravates the deleterious effects of the Southern bean mosaic virus in Rhizobium-nodulated Phaseolus vulgaris plants. Arch. Phytopathol. Plant Prot. 2010, 43, 1643–1657. [Google Scholar] [CrossRef]
- Brito, M.; Fernández-Rodríguez, T.; Garrido, M.J.; Mejías, A.; Romano, M.; Marys, E. First report of Cowpea mild mottle carlavirus on Yardlong bean (Vigna unguiculata subsp. sesquipedalis) in Venezuela. Viruses 2012, 4, 3804–3811. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, T.; Balogun, O.; Uddin, R. Cowpea virus disease occurrence: Implication for food security and sustainable development in Kwara State–Nigeria. Albanian J. Agric. Sci. 2013, 12, 633–639. [Google Scholar]
- Eni, A.O.; Ogunsanya, P.; Oviasuyi, T.; d’A Hughes, J. Alarming increase in the incidence of Cucumber mosaic virus in cowpea (Vigna unguiculata (L.) Walp.) in northern Nigeria. Arch. Phytopathol. Plant Prot. 2013, 46, 1958–1965. [Google Scholar] [CrossRef]
- Baranwal, V.K.; Jain, P.; Saritha, R.K.; Jain, R.K.; Gautam, N.K. Detection and partial characterization of Cowpea mild mottle virus in mungbean and urdbean by deep sequencing and RT-PCR. Crop Prot. 2015, 75, 77–79. [Google Scholar] [CrossRef]
- Gautam, N.K.; Kumar, K.; Prasad, M. Leaf crinkle disease in urdbean (Vigna mungo L. Hepper): An overview on causal agent, vector and host. Protoplasma 2016, 253, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, S.V.; Devadason, A.; Ganesan, M.V. Seed-borne nature of a begomovirus, Mung bean yellow mosaic virus in black gram. Appl. Microbiol. Biotechnol. 2016, 100, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.L.S.; Oliveira, J.T.A.; De Souza, G.A.; Vasconcelos, I.M. Label-free proteomics reveals that Cowpea severe mosaic virus transiently suppresses the host leaf protein accumulation during the compatible interaction with cowpea (Vigna unguiculata [L.] Walp.). J. Proteome Res. 2016, 15, 4208–4220. [Google Scholar] [CrossRef] [PubMed]
- Palanga, E.; Filloux, D.; Martin, D.P.; Fernandez, E.; Gargani, D.; Ferdinand, R.; Zabre, J.; Bouda, Z.; Neya, J.B.; Sawadogo, M.; et al. Metagenomic-based screening and molecular characterization of cowpea-infecting viruses in Burkina Faso. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzmiel, K.; Zarzyńska-Nowak, A.; Lewandowska, M.; Szydło, W. Identification of new Brome mosaic virus (BMV) isolates systemically infecting Vigna unguiculata L. Eur. J. Plant Pathol. 2016, 145, 233–238. [Google Scholar] [CrossRef]
- Vairam, N.; Lavanya, S.A.; Muthamilan, M.; Vanniarajan, C. Screening of M3 mutants for Yellow vein mosaic virus resistance in greengram [Vigna radiata (L.) Wilczek]. Int. J. Plant Sci. 2016, 11, 265–269. [Google Scholar] [CrossRef]
- Kundu, A.; Paul, S.; Dey, A.; Pal, A. High throughput sequencing reveals modulation of microRNAs in Vigna mungo upon Mungbean yellow mosaic India virus inoculation highlighting stress regulation. Plant Sci. 2017, 257, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.G.G.; Vasconcelos, I.M.; Martins, T.F.; Varela, A.L.N.; Souza, P.F.N.; Lobo, A.K.M.L.; Silva, F.D.A.; Silveira, J.A.G.; Oliveira, J.T.A. Drought increases cowpea (Vigna unguiculata [L.] Walp.) susceptibility to Cowpea severe mosaic virus (CPSMV) at early stage of infection. Plant Physiol. Biochem. 2016, 109, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, N.; Sah, R.P.; Shivakumar, M.S.; Archana, S. Effect of Bean common mosaic virus infection on yield potential and nodulation of cowpea genotypes. Range Manag. Agrofor. 2016, 37, 185–191. [Google Scholar]
- Chang, C.A.; Chien, L.Y.; Tsai, C.F.; Lin, Y.Y.; Cheng, Y.H. First report of Cowpea mild mottle virus in Cowpea and French bean in Taiwan. Plant Dis. 2013, 97, 7. [Google Scholar] [CrossRef]
- Almeida, Á.M.R.; Piuga, F.F.; Marin, S.R.R.; Kitajima, E.W.; Gaspar, J.O.; De Oliveira, T.G.; De Moraes, T.G. Detection and partial characterization of a carlavirus causing stem necrosis of soybean in Brazil. Fitopatol. Bras. 2005, 30, 191–194. [Google Scholar] [CrossRef]
- Rodrigues, J.C.V.; Kondidie, D.B.; Estevez-Jensen, C.; Kitajima, E.W.; Huckaba, R.M.; Foster, J.E. Infection in soybeans and on multiple host plants in Puerto Rico by an isolate of Cowpea mild mottle virus. Virus Rev. Res. 2014, 19, 4. [Google Scholar] [CrossRef]
- Zanardo, L.G.; Silva, F.N.; Bicalho, A.A.C.; Urquiza, G.P.C.; Lima, A.T.M.; Almeida, A.M.R.; Zerbini, F.M.; Carvalho, C.M. Molecular and biological characterization of Cowpea mild mottle virus isolates infecting soybean in Brazil and evidence of recombination. Plant Pathol. 2014, 63, 456–465. [Google Scholar] [CrossRef]
- Laguna, I.G.; Arneodo, J.D.; Rodríguez-Pardina, P.; Fiorona, M. Cowpea mild mottle virus infecting soybean crops in northwestern Argentina. Fitopatol. Bras. 2006, 31, 3. [Google Scholar] [CrossRef]
- Aliyu, T.H.; Balogun, O.S. Effects of variety and planting density on the incidence of common viral diseases of Cowpea (Vigna unguiculata) in a Southern Guinea Savannah agro-ecology. Asian J. Plant Pathol. 2011, 5, 126–133. [Google Scholar]
- Yadav, M.K.; Biswas, K.K.; Lal, S.K.; Baranwal, V.K.; Jain, R.K. A distinct strain of Cowpea mild mottle virus infecting soybean in India. J. Phytopathol. 2013, 161, 739–744. [Google Scholar] [CrossRef]
- Odedara, O.O.; Kumar, P.L. Incidence and diversity of viruses in cowpeas and weeds in the unmanaged farming systems of savanna zones in Nigeria. Arch. Phytopathol. Plant Prot. 2017, 50, 1–12. [Google Scholar] [CrossRef]
- Menzel, W.; Winter, S.; Vetten, H.J. Complete nucleotide sequence of the type isolate of Cowpea mild mottle virus from Ghana. Arch. Virol. 2010, 155, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Alegbejo, M.D. Whitefly transmitted plant viruses in Nigeria. J. Sustain. Agric. 2001, 17, 99–109. [Google Scholar] [CrossRef]
- Amayo, R.; Arinaitwe, A.B.; Mukasa, S.B.; Tusiime, G.; Kyamanywa, S.; Rubaihayo, P.R.; Edema, R. Prevalence of viruses infecting cowpea in Uganda and their molecular detection. Afr. J. Biotechnol. 2012, 11, 14132–14139. [Google Scholar]
- Regan, K.; Ordosch, D.; Glover, K.D.; Tilmon, K.J.; Szczepaniec, A. Effects of a pyrethroid and two neonicotinoid insecticides on population dynamics of key pests of soybean and abundance of their natural enemies. Crop Prot. 2017, 98, 24–32. [Google Scholar] [CrossRef]
- Wang, Y.; Janz, B.; Engedal, T.; de Neergaard, A. Effect of irrigation regimes and nitrogen rates on water use efficiency and nitrogen uptake in maize. Agric. Water Manag. 2017, 179, 271–276. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2016, 575, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, H.; Chen, L.; Zhang, H.; Gao, X. Induction of systemic disease resistance in Nicotiana benthamiana by the cyclodipeptides cyclo (l-Pro-l-Pro) and cyclo (d-Pro-d-Pro). Mol. Plant Pathol. 2016, 18, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T. Harnessing evolutionary toxins for signaling: Reactive oxygen species, nitric oxide and hydrogen sulfide in plant cell regulation. Front. Plant Sci. 2017, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, K.R.; Dallagnol, L.J.; Pazdiora, P.C.; Rodrigues, F.A.; Deuner, S. Silicon potentiates biochemical defense responses of wheat against tan spot. Physiol. Mol. Plant Pathol. 2017, 97, 69–78. [Google Scholar] [CrossRef]
- Njenga, K.W.; Nyaboga, E.; Wagacha, J.M.; Mwaura, F.B. Silicon induces resistance to bacterial blight by altering the physiology and antioxidant enzyme activities in cassava. World J. Agric. Res. 2017, 5, 42–51. [Google Scholar]
- Debona, D.; Rodrigues, F.; Datnoff, L. Silicon’s role in abiotic and biotic plant stress. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, C.H.; Kapoor, R.; Ganjewala, D. Alleviation of abiotic and biotic stresses in plants by silicon supplementation. Scientia 2016, 13, 59–73. [Google Scholar]
- Elsharkawy, M.M.; Mousa, K.M. Induction of systemic resistance against Papaya ring spot virus (PRSV) and its vector Myzus persicae by Penicillium simplicissimum GP17-2 and silica (SiO2) nanopowder. Int. J. Pest Manag. 2015, 61, 353–358. [Google Scholar] [CrossRef]
- Zellner, W.; Frantz, J.; Leisner, S. Silicon delays Tobacco ringspot virus systemic symptoms in Nicotiana tabacum. J. Plant Physiol. 2011, 168, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Mali, M.; Aery, N.C. Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata). J. Plant Nutr. Soil Sci. 2008, 171, 835–840. [Google Scholar] [CrossRef]
- Shen, X.; Li, Z.; Duan, L.; Eneji, A.E.; Li, J. Silicon mitigates ultraviolet-B radiation stress on soybean by enhancing chlorophyll and photosynthesis and reducing transpiration. J. Plant Nutr. 2014, 37, 837–849. [Google Scholar] [CrossRef]
- Kurdali, F.; Al-chammaa, M.; Mouasess, A. Growth and nitrogen fixation in silicon and/or potassium fed chickpeas grown under drought and well watered conditions. J. Stress Physiol. Biochem. 2013, 9, 385–406. [Google Scholar]
- Abu-muriefah, S.S. Effects of silicon on Faba bean (Vicia faba L.) plants grown under heavy metal stress conditions. African J. Agric. Sci. Technol. 2015, 3, 255–268. [Google Scholar]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E.; Gozukara, N. Genome-wide exploration of silicon (Si) transporter genes, Lsi1 and Lsi2 in plants; insights into Si-accumulation status/capacity of plants. BioMetals 2017, 30, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Leishman, M.R.; Hartley, S. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-G.; Fu, W.-Q.; Zhang, F.-M.; Shi, X.-M.; Zeng, Y.-T.; Li, H.; Zhang, W.; Dai, C.-C. The endophytic fungus Phomopsis liquidambari increases nodulation and N2 fixation in Arachis hypogaea by enhancing hydrogen peroxide and nitric oxide signalling. Microb. Ecol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Damiani, I.; Pauly, N.; Puppo, A.; Brouquisse, R.; Boscari, A. Reactive oxygen species and nitric oxide control early steps of the legume—Rhizobium symbiotic interaction. Front. Plant Sci. 2016, 7, 454. [Google Scholar] [CrossRef] [PubMed]
- Vandelle, E.; Ling, T.; Imanifard, Z.; Liu, R.; Delledonne, M.; Bellin, D. Chapter Eleven-Nitric Oxide Signaling during the Hypersensitive Disease Resistance Response. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2016; Volume 77, pp. 219–243. [Google Scholar]
- Thalineau, E.; Truong, H.-N.; Berger, A.; Fournier, C.; Boscari, A.; Wendehenne, D.; Jeandroz, S. Cross-regulation between N metabolism and nitric oxide (NO) signaling during plant immunity. Front. Plant Sci. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, Y.; Gu, Z.; Wang, R.; Sun, G.; Zhu, C.; Guo, S.; Shen, Q. Nitrate protects cucumber plants against Fusarium oxysporum by regulating citrate exudation. Plant Cell Physiol. 2016, 57, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, L.R.; Batista, B.L.; da Silva Lobato, A.K. Silicon reduces aluminum accumulation and mitigates toxic effects in cowpea plants. Acta Physiol. Plant. 2017, 39, 138. [Google Scholar] [CrossRef]
- Izaguirre-Mayoral, M.L.; Uzcategui, R.C.D.E.; Mallorca, M.S.D.E. Physiological and biochemical aspects of symbiotic nitrogen fixation in Cowpea (Vigna unguiculata (L.) Walp. var. Tuy) plants infected by Cowpea mosaic virus. J. Exp. Bot. 1992, 43, 455–462. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E.; Ryalls, J.M.W.; Frew, A.; BeGabriel, J.L.; Duncan, M.; Gherlenda, A.N. Silicon-induced root nodulation and synthesis of essential amino acids in a legume is associated with higher herbivore abundance. Funct. Ecol. 2017. [Google Scholar] [CrossRef]
- Ashfaque, F.; Inam, A.; Inam, A.; Iqbal, S.; Sahay, S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S. Afr. J. Bot. 2017, 111, 153–160. [Google Scholar] [CrossRef]
- Markovich, O.; Steiner, E.; Kouřil, Š.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and Sorghum. Plant Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Meilhoc, E.; Boscari, A.; Braund, C.; Frendo, P.; Brouquisse, R. Nitric oxide: Jack-of-all-trades of the nitrogen-fixing symbiosis? Adv. Bot. Res. 2016, 77, 193–218. [Google Scholar]
- Romero-Puertas, M.C.; Sandalio, L.M. Nitric oxide level is self-regulating and also regulates its ROS partners. Front. Plant Sci. 2016, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M. Transporters involved in source to sink partitioning of amino acids and ureides: Opportunities for crop improvement. J. Exp. Bot. 2014, 65, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Franklin, O.; Cambui, C.A.; Gruffman, L.; Palmroth, S.; Oren, R.; Näsholm, T. The carbon bonus of organic nitrogen enhances nitrogen use efficiency of plants. Plant Cell Environ. 2016, 40, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atilio, J.B.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Molero, G.; Tcherkez, G.; Araus, J.L.; Nogués, S.; Aranjuelo, I. On the relationship between C and N fixation and amino acid synthesis in nodulated alfalfa (Medicago sativa). Funct. Plant Biol. 2014, 41, 331–341. [Google Scholar] [CrossRef]
- Murray, J.D.; Liu, C.W.; Chen, Y.; Miller, A.J. Nitrogen sensing in legumes. J. Exp. Bot. 2017, 68, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.G.; Singh, A. Effect of nitrogen and zinc on nodulation, growth and yield of cowpea. Legum. Res. 2016, 39, 149–151. [Google Scholar]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Ashraf, I.; Ishque, W.; Faroog, M.U.; Usman, M.; Kharal, M. Effect of different nitrogen and phosphorus fertilizer levels in combination with nitrogen and phosphorus solubilizing inoculants on the growth and yield of mung bean. Pakistan J. Life Soc. Sci. 2017, 15, 31–36. [Google Scholar]
- Martelli, G.P.; Saldarelli, P. Carlavirus. In The Springer Index of Viruses; Springer: New York, NY, USA, 2011; pp. 521–532. [Google Scholar]
- Fujita, N.; Komatsu, K.; Ayukawa, Y.; Matsuo, Y.; Hashimoto, M.; Netsu, O.; Teraoka, T.; Yamaji, Y.; Namba, S.; Arie, T. N-terminal region of cysteine-rich protein (CRP) in carlaviruses is involved in the determination of symptom types. Mol. Plant Pathol. 2016, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, E.A.; Lezzhov, A.A.; Komarova, T.V.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. A novel block of plant virus movement genes. Mol. Plant Pathol. 2017, 18, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Harak, C.; Lohmann, V. Ultrastructure of the replication sites of positive-strand RNA viruses. Virology 2015, 479–480, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Romero-Brey, I.; Bartenschlager, R. Endoplasmic reticulum: The favorite intracellular niche for viral replication and assembly. Viruses 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Rao, A.L.N. Live cell imaging of interactions between replicase and capsid protein of Brome mosaic virus using bimolecular fluorescence complementation: Implications for replication and genome packaging. Virology 2014, 464–465, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, J.; Labbe, C.; Menzies, J.G.; Belanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef] [PubMed]
- El-Shetehy, M.; Wang, C.; Shine, M.B.; Yu, K.; Kachroo, A.; Kachroo, P. Nitric oxide and reactive oxygen species are required for systemic acquired resistance in plants. Plant Signal. Behav. 2015, 10, e998544. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Zhang, D.; Zhu, F.; Wang, S.; Zhu, T.; Pu, X.; Zheng, T.; Feng, H.; Lin, H. Nitrate reductase-dependent nitric oxide production is required for regulation alternative oxidase pathway involved in the resistance to Cucumber mosaic virus infection in Arabidopsis. Plant Growth Regul. 2015, 77, 99–107. [Google Scholar] [CrossRef]
- Singh, A.; Lim, G.H.; Kachroo, P. Transport of chemical signals in systemic acquired resistance. J. Integr. Plant Biol. 2017, 59, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.G.; Zhu, T.; Zou, L.J.; Han, X.Y.; Zhou, X.; Xi, D.H.; Zhang, D.W.; Lin, H.H. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016, 85, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Prudent, M.; Vernoud, V.; Girodet, S.; Salon, C. How nitrogen fixation is modulated in response to different water availability levels and during recovery: A structural and functional study at the whole plant level. Plant Soil 2016, 399, 1–12. [Google Scholar] [CrossRef]
- Weintraub, M. A Carlavirus and a Rhabdovirus infecting Lonicera × brownii cv. Dropmore Scarlet in Western Canada. J. Phytopathol. 1993, 139, 57–67. [Google Scholar] [CrossRef]
- Gaspar, J.O.; Costa, A.S. Effect of Bean angular mosaic virus on carbohydrate metabolism of jalo bean. Fitopatol. Bras. 1993, 18, 541–544. [Google Scholar]
- Kim, K.S. An ultrastructural study of inclusions and disease development in plant cells infected by Cowpea chlorotic mottle virus. J. Gen. Virol. 1977, 35, 535–543. [Google Scholar] [CrossRef]
- Llave, C. Dynamic cross-talk between host primary metabolism and viruses during infections in plants. Curr. Opin. Virol. 2016, 19, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Phansak, P.; Taylor, P.W.J.; Mongkolporn, O. Genetic diversity in yardlong bean (Vigna unguiculata ssp. sesquipedalis) and related Vigna species using sequence tagged microsatellite site analysis. Sci. Hortic. 2005, 106, 137–146. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izaguirre-Mayoral, M.L.; Brito, M.; Baral, B.; Garrido, M.J. Silicon and Nitrate Differentially Modulate the Symbiotic Performances of Healthy and Virus-Infected Bradyrhizobium-nodulated Cowpea (Vigna unguiculata), Yardlong Bean (V. unguiculata subsp. sesquipedalis) and Mung Bean (V. radiata). Plants 2017, 6, 40. https://doi.org/10.3390/plants6030040
Izaguirre-Mayoral ML, Brito M, Baral B, Garrido MJ. Silicon and Nitrate Differentially Modulate the Symbiotic Performances of Healthy and Virus-Infected Bradyrhizobium-nodulated Cowpea (Vigna unguiculata), Yardlong Bean (V. unguiculata subsp. sesquipedalis) and Mung Bean (V. radiata). Plants. 2017; 6(3):40. https://doi.org/10.3390/plants6030040
Chicago/Turabian StyleIzaguirre-Mayoral, Maria Luisa, Miriam Brito, Bikash Baral, and Mario José Garrido. 2017. "Silicon and Nitrate Differentially Modulate the Symbiotic Performances of Healthy and Virus-Infected Bradyrhizobium-nodulated Cowpea (Vigna unguiculata), Yardlong Bean (V. unguiculata subsp. sesquipedalis) and Mung Bean (V. radiata)" Plants 6, no. 3: 40. https://doi.org/10.3390/plants6030040
APA StyleIzaguirre-Mayoral, M. L., Brito, M., Baral, B., & Garrido, M. J. (2017). Silicon and Nitrate Differentially Modulate the Symbiotic Performances of Healthy and Virus-Infected Bradyrhizobium-nodulated Cowpea (Vigna unguiculata), Yardlong Bean (V. unguiculata subsp. sesquipedalis) and Mung Bean (V. radiata). Plants, 6(3), 40. https://doi.org/10.3390/plants6030040