Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects
Abstract
:1. Introduction
2. Drought Stress and its Impacts on Plant Physiology
3. Role of Melatonin in Regulation of Plant Physiology
4. Melatonin Mediated Regulation of Plant Biology under Drought Stress
4.1. Regulation of Photosynthetic Response
4.2. Regulation of Oxidative Stress and Antioxidative Defense System
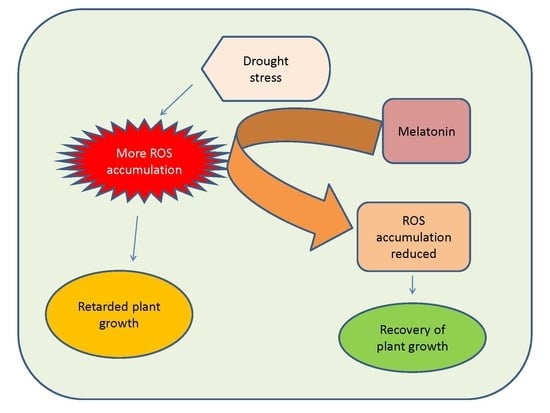
4.2.1. Impact on ROS Accumulation
4.2.2. Impact on Enzymatic and Non-Enzymatic Anti-oxidative Defense System
4.3. Regulation of Other Biological Processes Related to Drought Tolerance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.X.; Xin, L.F.; Zheng, H.F.; Li, L.L.; Ran, W.L.; Mao, J.; Yang, Q.H. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica 2016, 54, 74–80. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Ying, Y.; Yue, Y.; Huang, X.; Wang, H.; Mei, L.; Yu, W.; Zheng, B.; Wu, J. Salicylic acid induces physiological and biochemical changes in three Red bayberry (Myric rubra) genotypes under water stress. Plant Growth Regul. 2013, 71, 181–189. [Google Scholar] [CrossRef]
- Cao, B.-L.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci. Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Kachroo, P. Salicylic acid-, jasmonic acid- and ethylene-mediated regulation of plant defense signaling. Genet. Eng. 2007, 28, 55–83. [Google Scholar]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Hong, C.; Chen, H.; Zeng, F.; Zheng, B.; Jiang, D. Malate secretion from the root system is an important reason for higher resistance of Miscanthus sacchariflorus to cadmium. Physiol. Plant. 2017, 159, 340–353. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Khan, T.A.; Yusuf, M.; Ahmad, A.; Bashir, Z.; Saeed, T.; Fariduddin, Q.; Hayat, S.; Mock, H.P.; Wu, T. Proteomic and physiological assessment of stress sensitive and tolerant variety of tomato treated with brassinosteroids and hydrogen peroxide under low-temperature stress. Food Chem. 2019, 289, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.U.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Kumar, V.; Kaur, P.; Bhardwaj, R. Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk. J. Biol. 2017, 41, 943–953. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R. 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol. 2017, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Ahmad, Z.; Anjum, S.; Waraich, E.A.; Ayub, M.A.; Ahmad, T.; Tariq, R.M.S.; Ahmad, R.; Iqbal, M.A. Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress—A review. J. Plant Nutr. 2018, 41, 1734–1743. [Google Scholar] [CrossRef]
- Foyer, C.; Noctor, G. Oxygen processing in photosynthesis: Regulation and signaling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnam, B.; Iuchi, S.; Fujita, M.; Fujita, Y.; Takasaki, H.; Osakabe, Y.; Yamaguchi-Shinozaki, K.; Kobayashi, M.; Shinozaki, K. Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 2013, 20, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Scippa, G.S.; Di Michele, M.; Onelli, E.; Patrignani, G.; Chiatante, D.; Bray, E.A. The histone-like protein H1-S and the response of tomato leaves to water deficit. J. Exp. Bot. 2004, 55, 99–109. [Google Scholar] [CrossRef] [PubMed]
- de Campos, M.K.F.; de Carvalho, K.; de Souza, F.S.; Marur, C.J.; Pereira, L.F.P.; Filho, J.C.B.; Vieira, L.G.E. Drought tolerance and antioxidant enzymatic activity in transgenic ‘Swingle’ citrumelo plants over-accumulating proline. Environ. Exp. Bot. 2011, 72, 242–250. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Andersen, M.N. Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: Its implication in altering pod set. Field Crops Res. 2004, 86, 1–13. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Adak, M.S.; Bagci, E.G.; Cicek, N.; Eraslan, F. Effect of drought stress implemented at pre- or post-anthesis stage on some physiological parameters as screening criteria in chickpea cultivars. Russ. J. Plant Physiol. 2007, 55, 59–67. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. Potassium availability in relation to soil moisture. Plant Soil 1986, 95, 221–231. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.P.D.; Milach, S.C.K. Cold tolerance at the germination stage of rice: Methods of evaluation and characterization of genotypes. Sci. Agric. 2004, 61, 1–8. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought Induces Oxidative Stress and Enhances the Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nam, N.H.; Chauhan, Y.S.; Johansen, C. Osmotic adjustment, water relations and carbohydrate remobilization in pigeonpea under water deficits. J. Plant Physiol. 2000, 157, 651–659. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.-J.; Siddique, K.H.J.C.; Science, P. Chilling tolerance in maize: Agronomic and physiological approaches. Crop Pasture Sci. 2009, 60, 501–516. [Google Scholar] [CrossRef]
- Kiani, S.P.; Talia, P.; Maury, P.; Grieu, P.; Heinz, R.; Perrault, A.; Nishinakamasu, V.; Hopp, E.; Gentzbittel, L.; Paniego, N.; et al. Genetic analysis of plant water status and osmotic adjustment in recombinant inbred lines of sunflower under two water treatments. Plant Sci. 2007, 172, 773–787. [Google Scholar] [CrossRef]
- Chimenti, C.A.; Marcantonio, M.; Hall, A.J. Divergent selection for osmotic adjustment results in improved drought tolerance in maize (Zea mays L.) in both early growth and flowering phases. Field Crops Res. 2006, 95, 305–315. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Murch, S.J.; Campbell, S.S.B.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of Hypericum perforatum L. In Vitro Cell. Dev. Biol. Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Melatonin: A potential regulator of plant growth and development? In Vitro Cell. Dev. Biol. Plant 2002, 38, 531–536. [Google Scholar] [CrossRef]
- Park, S.; Back, K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 2012, 53, 385–389. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, E.; Therios, I.; Dimassi-Theriou, K. Melatonin and other factors that promote rooting and sprouting of shoot cuttings in Punica granatum cv. Wonderful. Turk. J. Bot. 2014, 38, 293–301. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Koyama, F.C.; Carvalho, T.L.; Alves, E.; da Silva, H.B.; de Azevedo, M.F.; Hemerly, A.S.; Garcia, C.R. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. J. Eukaryot. Microbiol. 2013, 60, 646–651. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Munoz-Parra, E.; Ortiz-Castro, R.; Lopez-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, D.X.; Jiang, D.; Liu, F. Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Reiter, R.J.; et al. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Zuo, Z.Y.; Sun, L.Y.; Wang, T.Y.; Miao, P.; Zhu, X.C.; Liu, S.Q.; Song, F.B.; Mao, H.P.; Li, X.N. Melatonin Improves the Photosynthetic Carbon Assimilation and Antioxidant Capacity in Wheat Exposed to Nano-ZnO Stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Janas, K.M. Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiol. Plant. 2007, 29, 509–517. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Janas, K.M.; Posmyk, M.M. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol. Plant. 2013, 35, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, N.; Wang, J.; Cao, Y.; Li, X.; Zhang, H.; Zhang, L.; Tan, D.X.; Guo, Y.D. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J. Pineal Res. 2016, 61, 138–153. [Google Scholar] [CrossRef]
- Karaca, P.; Cekic, F.Ö. Exogenous melatonin-stimulated defense responses in tomato plants treated with polyethylene glycol. Int. J. Veg. Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Schelbert, S.; Park, S.Y.; Han, S.H.; Lee, B.D.; Andres, C.B.; Kessler, F.; Hortensteiner, S.; Paek, N.C. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Nakajima, T.; Shigyo, M.; Yamauchi, N. Pheophytinase activity and gene expression of chlorophyll-degrading enzymes relating to UV-B treatment in postharvest broccoli (Brassica oleracea L. Italica Group) florets. Postharvest Biol. Technol. 2012, 63, 60–66. [Google Scholar] [CrossRef]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Yuan, H.; Kanwar, M.K.; Bhardwaj, R.; Thukral, A.K.; Zheng, B. Jasmonic Acid Seed Treatment Stimulates Insecticide Detoxification in Brassica juncea L. Front. Plant Sci. 2018, 9, 1609. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef] [PubMed]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 2017, 203, 286–294. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous Melatonin Improves Tolerance to Water Deficit by Promoting Cuticle Formation in Tomato Plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.J.; Du, Y.T.; Zhou, Y.B.; Chen, J.; Xu, Z.S.; Ma, Y.Z.; Chen, M.; Min, D.H. Overexpression of TaCOMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, L.; Cheng, Y.; Lu, G.; Fu, G.; Ma, H.; Liu, Q.; Zhang, X.; Zou, X.; Li, C. Exogenous melatonin alleviates damage from drought stress in Brassica napus L. (rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 43. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Bela, K.; Horvath, E.; Galle, A.; Szabados, L.; Tari, I.; Csiszar, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Hell, R.; Bergmann, L. λ-Glutamylcysteine synthetase in higher plants: Catalytic properties and subcellular localization. Planta 1990, 180, 603. [Google Scholar] [CrossRef]
- Subbarao, G.; Chauhan, Y.; Johansen, C. Patterns of osmotic adjustment in pigeonpea—Its importance as a mechanism of drought resistance. Eur. J. Agron. 2000, 12, 239–249. [Google Scholar] [CrossRef]
- Signorelli, S. The Fermentation Analogy: A Point of View for Understanding the Intriguing Role of Proline Accumulation in Stressed Plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, D.; Li, S.; Gao, Z.; Zhao, S.; Shi, J.; Wu, C.; Guo, X. A cotton group C MAP kinase gene, GhMPK2, positively regulates salt and drought tolerance in tobacco. Plant Mol. Biol. 2011, 77, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Cao, Y.; Qi, C.; Li, S.; Liu, L.; Wang, G.; Mao, A.; Ren, S.; Guo, Y.D. CsATAF1 Positively Regulates Drought Stress Tolerance by an ABA-Dependent Pathway and by Promoting ROS Scavenging in Cucumber. Plant Cell Physiol. 2018, 59, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Hoang, X.L.T.; Nhi, D.N.H.; Thu, N.B.A.; Thao, N.P.; Tran, L.P. Transcription Factors and Their Roles in Signal Transduction in Plants under Abiotic Stresses. Curr. Genom. 2017, 18, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological responses of forest trees to heat and drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Zhou, K.; Sun, T.; Hu, L.; Li, C.; Ma, F. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef]



| Plant Name | Conc. | Impact on Photosynthetic Parameters under Drought Stress | Reference |
|---|---|---|---|
| Actinidia chinesis | 100 µM | Recovery of leaf area, chlorophyll and carotenoid contents, photochemical efficiency of PSII along with photosynthetic electron transport rate. Better photosynthetic rate, stomatal conductance and transpiration rate. | [31] |
| Agrostis stolonifera | 20 µM | Recovery in relative water content, chlorophyll content and photochemical efficiency. Down-regulation of genes involved in chlorophyll degradation like CHLASE, PPH and CHL-PRX. | [11] |
| Coffea arabica | 300 µM | Better leaf water potential. Increased chlorophyll content, photosynthetic rate, stomatal conductance and transpiration rate. | [29] |
| Cucumis sativus | 100 µM | Improved photosynthetic rate, stomatal conductance, chlorophyll content and photochemical efficiency of PSII. | [70] |
| Dracocephalum moldavica | 100 µM | Recovery of chlorophyll content accompanied by increased leaf length and leaf area. | [27] |
| Malus domestica | 100 µM | Recovery of chlorophyll content accompanied by down-regulation of transcript levels of chlorophyll degrading enzyme PAO. Improved photosynthetic rate, stomatal conductance, and photochemical efficiency of PSII along with photosynthetic electron transport rate. | [32] |
| Increased chlorophyll content, photosynthetic rate, stomatal conductance and transpiration rate. Increased length, width and aperture of stomata. | [51] | ||
| Malus prunifolia and M. hupehensis | 100 µM | Better relative water content. Improved photosynthetic rate, stomatal conductance and chlorophyll content. Recovery of stomatal opening along with improved stomatal length, width and aperture. | [96] |
| Solanum lycopersicum | 200 µM | Recovery of chlorophyll content. | [88] |
| 100 µM | Better cell wall stability accompanied by less leaching of chlorophyll molecules. | [97] | |
| 0.1 mM | Improved photosynthetic rate, stomatal conductance, chlorophyll content and photochemical efficiency of PSII. | [98] | |
| Triticum aestivum | 100 µM | Recovery of chloroplast apparatus, photosynthetic rate, stomatal conductance, transpiration rate and photochemical efficiency of PSII. | [30] |
| Vitis vinifera | 100 nM | Increased chlorophyll content and photochemical efficiency. Recovery of damaged chloroplast ultrastructure and stomata. | [6] |
| Zea mays | 1 mM | Recovery of photochemical efficiency of PSII. | [94] |
| 100 µM | Better leaf area accompanied by recovery in chlorophyll content, photosynthetic rate, stomatal conductance and transpiration rate. Improved water potential, photochemical efficiency of PSII along with photosynthetic electron transport rate. | [42] |
| Plant Name | Conc. | Impact on Oxidative Stress Markers under Drought Stress | Reference |
|---|---|---|---|
| Actinidia chinesis | 100 µM | Reduction in MDA content and membrane injury index. | [31] |
| Agrostis stolonifera | 20 µM | Reduction in contents of H2O2 and MDA accompanied by declined electrolyte leakage. | [11] |
| Avena nuda | 100 µM | Reduction in contents of superoxide anion and H2O2. | [99] |
| Brassica napus | 50 µM | Reduction in H2O2 content. | [103] |
| Coffea arabica | 300 µM | Reduction in lipid peroxidation. | [29] |
| Cucumis sativus | 100 µM | Reduction in contents of H2O2, hydroxyl radical and MDA accompanied by declined electrolyte leakage. | [70] |
| Dracocephalum moldavica | 100 µM | Reduction in contents of H2O2, MDA and other aldehydes which cause oxidative stress accompanied by declined electrolyte leakage. | [2] |
| Malus domestica | 100 µM | Reduction in electrolyte leakage accompanied by declined H2O2 content. | [51] |
| Reduction in H2O2 content. | [32] | ||
| Malus prunifolia and M. hupehensis | 100 µM | Reduction in H2O2 content. Accumulation of ABA is reduced. | [96] |
| Solanum lycopersicum | 200 µM | Reduction in lipid peroxidation. | [88] |
| 0.1 mM | Reduction in contents of superoxide anion and MDA. | [98] | |
| Triticum aestivum | 100 µM | Reduction in contents of superoxide anion, H2O2 and MDA accompanied by declined electrolyte leakage. | [30] |
| Vitis vinifera | 100 nM | Reduction in contents of superoxide anion and H2O2. | [6] |
| Zea mays | 100 µM | Reduction in contents of H2O2 and MDA. Better DPPH scavenging activity. | [42] |
| Plant Name | Conc. | Impact on Antioxidative Enzymes under Drought Stress | Reference |
|---|---|---|---|
| Avena nuda | 100 µM | Enhanced activities of APX, CAT, POD and SOD. | [99] |
| Brassica napus | 50 µM | Enhanced activities of APX, CAT and POD. | [103] |
| Coffea arabica | 300 µM | Enhanced activities of APX and CAT, but no significant difference in SOD activity. | [29] |
| Cucumis sativus | 100 µM | Enhanced activities of CAT, POD and SOD. | [70] |
| Dracocephalum moldavica | 100 µM | Enhanced activities of APX, CAT, GPX and SOD. | [27] |
| Malus domestica | 100 µM | Enhanced activities of APX, CAT, POD, DHAR, MDHAR and GR. | [32] |
| Malusprunifolia and M. hupehensis | 100 µM | Enhanced activities of APX, CAT and POD. | [96] |
| Solanum lycopersicum | 200 µM | Enhanced GR activity. | [88] |
| 0.1 mM | Enhanced activities of APX, CAT, GR, POD and SOD. | [98] | |
| Triticum aestivum | 100 µM | Enhanced activities of APX, GPX, DHAR, MDHAR, GST and GR. Up-regulation in the transcript levels of APX, DHAR, MDHAR4, GPX, GPX1, GR and GST2. | [30] |
| Vitis vinifera | 100 nM | Enhanced activities of CAT, POD and SOD. | [6] |
| Zea mays | 100 µM | Enhanced activities of APX, CAT, POD and SOD. | [42] |
| Plant Name | Conc. | Impact on Non-Enzymatic Antioxidants under Drought Stress | Reference |
|---|---|---|---|
| Actinidia chinesis | 100 µM | Accumulation of soluble sugars and proline is increased. This is accompanied by better cellular osmotic adjustments, resulting in reduction of cell injury. | [31] |
| Brassica napus | 50 µM | Increased accumulation of total soluble sugars and proline, accompanied by better osmotic regulation capacity. | [103] |
| Coffea arabica | 300 µM | Increased accumulation of sucrose, total soluble sugars, ascorbate and proline, accompanied by improvement in leaf water potential. | [29] |
| Dracocephalum moldavica | 100 µM | Increased accumulation of proline accompanied by better relative water content. | [27] |
| Malus domestica | 100 µM | Increased accumulation of GSH, total GSH, AsA and total AsA. Reduction in DHA and GSSG accumulation, accompanied by higher ratios of GSH/GSSG and AsA/DHA. | [32] |
| Solanum lycopersicum | 200 µM | Accumulation of p-coumaric acid (a phenolic compound) is increased under only melatonin treatment. | [88] |
| 0.1 mM | Increased accumulation total AsA. | [98] | |
| Triticum aestivum | 100 µM | Increased accumulation of GSH, total GSH, AsA and total AsA. Reduction in DHA accumulation.Higher ratios of GSH/GSSG and AsA/DHA.Better cell turgor accompanied by improved water holding capacity leads to osmotic adjustments in drought stressed cells. | [30] |
| Vitis vinifera | 100 nM | Accumulation of ascorbate, glutathione and proline is enhanced. Proline is suggested to be involved in regulation of osmotic potential of drought stressed cells. | [6] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8, 190. https://doi.org/10.3390/plants8070190
Sharma A, Zheng B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants. 2019; 8(7):190. https://doi.org/10.3390/plants8070190
Chicago/Turabian StyleSharma, Anket, and Bingsong Zheng. 2019. "Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects" Plants 8, no. 7: 190. https://doi.org/10.3390/plants8070190
APA StyleSharma, A., & Zheng, B. (2019). Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants, 8(7), 190. https://doi.org/10.3390/plants8070190