Molecular and Biochemical Differences in Leaf Explants and the Implication for Regeneration Ability in Rorippa aquatica (Brassicaceae)
Abstract
:1. Introduction
2. Results
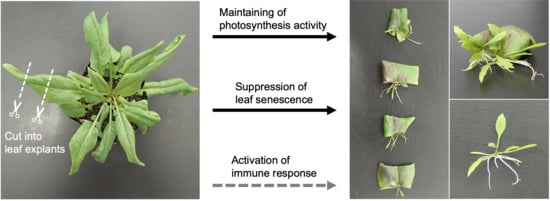
2.1. Leaf Explants of R. aquatica Are Highly Viable
2.2. Photosynthesis Is Required for the Survival of Leaf Explants and Plantlet Regeneration in R. aquatica
2.3. Phytohormones Are Regulated Differently in R. aquatica and A. thaliana Leaf Explants
2.4. Senescence and Immune Responses Are Regulated at the Transcriptional Level in R. aquatica Leaf Explants
2.5. Exogenous ABA and JA Affect the Efficiency of Plantlet Regeneration
2.6. Internal Leaf Structure May Affect the Viability of Leaf Explants
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Plantlet Regeneration
5.2. Photosynthetic Activity Measurement
5.3. Quantification of Phytohormones
5.4. Photosynthetic Inhibitor and Phytohormone Supplementation
5.5. Analysis of Gene Expression Profiles Using Transcriptome Data
5.6. Observation of the Internal Structure of Rosette Leaves
5.7. Statistical Analysis
5.8. Sequence Data
Author Contributions
Funding
Conflicts of Interest
References
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R. Hartmann and Kester’s Plant Propagation: Principles and Practices, 7th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2002; pp. 277–291. [Google Scholar]
- Amano, R.; Nakayama, H.; Momoi, R.; Omata, E.; Gunji, S.; Takebayashi, Y.; Kojima, M.; Ikematsu, S.; Ikeuchi, M.; Iwase, A.; et al. Molecular basis for natural vegetative propagation via regeneration in North American Lake cress, Rorippa aquatica (Brassicaceae). Plant Cell Physiol. 2020, 61, 353–369. [Google Scholar] [CrossRef]
- La Rue, C.D. Regeneration in Radicula aquatica. Mich. Acad. 1943, 28, 51–61. [Google Scholar]
- Nakayama, H.; Nakayama, N.; Seiki, S.; Kojima, M.; Sakakibara, H.; Sinha, N.; Kimura, S. Regulation of the KNOX-GA gene module induces heterophilic alteration in North American lake cress. Plant Cell 2014, 26, 4733–4748. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Fukushima, K.; Fukuda, T.; Yokoyama, J.; Kimura, S. Molecular phylogeny determined using chloroplast DNA inferred a new phylogenetic relationship of Rorippa aquatica (Eaton) EJ Palmer & Steyermark (Brassicaceae)–Lake Cress. AJPS 2014, 5, 48–54. [Google Scholar]
- Metz, J.G.; Pakrasi, H.B.; Seibert, M.; Arntzen, C.J. Evidence for a dual function of the herbicide-binding D1 protein photosystem II. FEBS Lett. 1986, 205, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Gan, S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, W.; Liu, L.; Chen, T.; Zhou, F.; Lin, Y. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biol. 2013, 13, 132. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Worley, E.; Udvardi, M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 2014, 26, 4862–4874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Xia, X.; Zhang, Y.; Gan, S.S. An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J. 2012, 69, 667–678. [Google Scholar] [CrossRef]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chetelat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Calvo, P.; Chini, A.; Fernandez-Barbero, G.; Chico, J.M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Chu, C. Towards understanding abscisic acid-mediated leaf senescence. Sci. China Life. Sci. 2015, 58, 506–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Gan, S.S. An Abscisic Acid-AtNAP Transcription Factor-SAG113 Protein Phosphatase 2C Regulatory Chain for Controlling Dehydration in Senescing Arabidopsis Leaves. Plant Physiol. 2012, 58, 961–969. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.; Kato, J. Isolation and identification of a senescence-promoting substance from wormwood (Artemisia absinthium L.). Plant Physiol. 1980, 66, 246–249. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.A.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef] [Green Version]
- Chini, A.; Fonseca, S.; Fernandez, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; Garcia-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Chen, L.; Tong, L.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.W.; Xu, L. YUCCA-mediated auxin biosynthesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Sakamoto, T.; Okegawa, Y.; Kaminoyama, K.; Fujiie, M.; Ichihashi, Y.; Kurata, T.; Motohashi, K.; Al-Shehbaz, I.; Sinha, N.; et al. Comparative transcriptomics with self-organizing map reveals cryptic photosynthetic differences between two accessions of North American Lake cress. Sci. Rep. 2018, 8, 3302. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kojima, M.; Kamada-Nobusada, T.; Komatsu, H.; Takei, K.; Kuroha, T.; Mizutani, M.; Ashikari, M.; Ueguchi-Tanaka, M.; Matsuoka, M.; Suzuki, K.; et al. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: An application for hormone profiling on Oryza sativa. Plant Cell Physiol. 2009, 50, 1201–1214. [Google Scholar] [CrossRef]
- Kojima, M.; Sakakibara, H. Highly sensitive high-throughput profiling of six phytohormones using MS-probe modification and liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 2012, 918, 151–164. [Google Scholar]
- Mashiguchi, K.; Hisanno, H.; Takeda–Kamiya, N.; Takebayashi, Y.; Ariizumi, T.; Gao, Y.; Ezura, H.; Sato, K.; Zhao, Y.; Hayashi, K.I.; et al. Agrobacterium tumefaciens enhances biosynthesis of two distinct auxins in the formation of crown galls. Plant Cell Physiol. 2019, 60, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Tsukaya, H.; Naito, S.; Rédei, G.P.; Komeda, Y. A new class of mutations in Arabidopsis thaliana, acaulis1, affecting the development of both inflorescences and leaves. Development 1993, 118, 751–764. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amano, R.; Momoi, R.; Omata, E.; Nakahara, T.; Kaminoyama, K.; Kojima, M.; Takebayashi, Y.; Ikematsu, S.; Okegawa, Y.; Sakamoto, T.; et al. Molecular and Biochemical Differences in Leaf Explants and the Implication for Regeneration Ability in Rorippa aquatica (Brassicaceae). Plants 2020, 9, 1372. https://doi.org/10.3390/plants9101372
Amano R, Momoi R, Omata E, Nakahara T, Kaminoyama K, Kojima M, Takebayashi Y, Ikematsu S, Okegawa Y, Sakamoto T, et al. Molecular and Biochemical Differences in Leaf Explants and the Implication for Regeneration Ability in Rorippa aquatica (Brassicaceae). Plants. 2020; 9(10):1372. https://doi.org/10.3390/plants9101372
Chicago/Turabian StyleAmano, Rumi, Risa Momoi, Emi Omata, Taiga Nakahara, Kaori Kaminoyama, Mikiko Kojima, Yumiko Takebayashi, Shuka Ikematsu, Yuki Okegawa, Tomoaki Sakamoto, and et al. 2020. "Molecular and Biochemical Differences in Leaf Explants and the Implication for Regeneration Ability in Rorippa aquatica (Brassicaceae)" Plants 9, no. 10: 1372. https://doi.org/10.3390/plants9101372
APA StyleAmano, R., Momoi, R., Omata, E., Nakahara, T., Kaminoyama, K., Kojima, M., Takebayashi, Y., Ikematsu, S., Okegawa, Y., Sakamoto, T., Kasahara, H., Sakakibara, H., Motohashi, K., & Kimura, S. (2020). Molecular and Biochemical Differences in Leaf Explants and the Implication for Regeneration Ability in Rorippa aquatica (Brassicaceae). Plants, 9(10), 1372. https://doi.org/10.3390/plants9101372