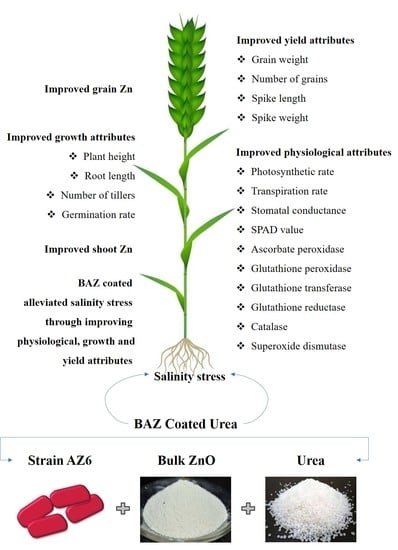
Impact of Coating of Urea with Bacillus-Augmented Zinc Oxide on Wheat Grown under Salinity Stress
Abstract
:1. Introduction
2. Results
2.1. Soil Characterization
2.2. Physiological Attributes of Wheat
2.3. Improvement in Growth Attributes
2.4. Effect on Yield Parameters
2.5. Antioxidant Assays
2.6. Impact on Macro- and Micronutrient Content of Wheat
2.7. Relationship and Variation among Morpho-Physio and Biochemical Attributes of Wheat
3. Discussion
4. Materials and Methods
4.1. Preparation of Bacillus sp. Strain AZ6 Inoculum
4.2. Production of Bacillus Augmented ZnO (BAZ)-Coated Urea
4.3. Soil Characterization
4.4. Experimental Setup
4.5. Physiological Measurements
4.6. Determination of Growth Attributes
4.7. Antioxidant Enzyme Activity
4.8. Measurement of Wheat Yield
4.9. Analysis of Macro- and Micronutrients
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ausubel, J.H.J.; Wernick, I.K.I.; Waggoner, P.E.P. Peak farmland and the prospect for land sparing. Popul. Dev. Rev. 2013, 38, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Paz, A.M.; Castanheira, N.; Farzamian, M.; Paz, M.C.; Gonçalves, M.C.; Santos, F.A.; Triantafilis, J. Prediction of soil salinity and sodicity using electromagnetic conductivity imaging. Geoderma 2020, 361, 114086. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; De Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, M.; Mishra, J.; Mishra, V. Environmental sustainability: Challenges and viable solutions. Environ. Sustain. 2018, 1, 309–340. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Hou, M.; Qiu, Y.; Zhang, T.; Yuan, Y. Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma 2020, 357, 113961. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanveer, M.; Ahmed, H.A. ROS Signalling in modulating salinity stress tolerance in plants. In Salt and Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020; pp. 299–314. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and function of zinc in plants. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherland, 1993; pp. 90–106. [Google Scholar]
- Sinclair, S.A.; Kramer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta 2012, 1823, 1553–1567. [Google Scholar] [CrossRef]
- Chandel, G.; Banerjee, S.; See, S.; Meena, R.; Sharma, D.J.; Verulkar, S.B. Effects of different nitrogen fertilizer levels and native soil properties on rice grain Fe, Zn and protein contents. Rice Sci. 2010, 17, 213–227. [Google Scholar] [CrossRef]
- Dimkpa, C.; Bindraban, P.S. Micronutrients fortification for efficient agronomic production: A review. Agron. Sustain. Dev. 2016, 36, 7. [Google Scholar] [CrossRef] [Green Version]
- Bindraban, P.S.; Dimkpa, C.O.; White, J.C.; Franklin, F.A.; Melse-Boonstra, A.; Koele, N.; Pandey, R.; Rodenburg, J.; Senthilkumar, K.; Demokritou, P.; et al. Safeguarding human and planetary health demands a fertilizer sector transformation. Plants People Planet 2020. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium; International Fertilizer Industry Association Paris: Paris, France, 2008. [Google Scholar]
- Amiri, A.; Baninasab, B.; Ghobadi, C.; Khoshgoftarmanesh, A.H. Zinc soil application enhances photosynthetic capacity and antioxidant enzyme activities in almond seedlings affected by salinity stress. Photosynthetica 2016, 54, 267–274. [Google Scholar] [CrossRef]
- Azarmi, F.; Mozafari, V.; Dahaji, P.A.; Hamidpour, M. Biochemical, physiological and antioxidant enzymatic activity responses of pistachio seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol. Plant. 2016, 38, 21. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Singapore, 2019; pp. 225–237. [Google Scholar]
- Hidri, R.; Mahmoud, O.M.; Debez, A.; Abdelly, C.; Barea, J.M.; Azcon, R. Modulation of C: N: P stoichiometry is involved in the effectiveness of a PGPR and AM fungus in increasing salt stress tolerance of Sulla carnosa Tunisian provenances. Appl. Soil Ecol. 2019, 143, 161–172. [Google Scholar] [CrossRef]
- Hafeez, F.Y.; Abaid-Ullah, M.; Hassan, M.N. Plant growth-promoting rhizobacteria as zinc mobilizers: A promising approach for cereals biofortification. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; pp. 217–235. [Google Scholar]
- Dimkpa, C.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Arshad, M.; Zahir, Z.A.; Asghar, M. Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak. J. Agric. Sci. 2015, 52, 915–922. [Google Scholar]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Imran, M.; Ahmad, M.; Hussain, S. Integrating the potential of Bacillus sp. Az6 and organic waste for zinc oxide bio-activation to improve growth, yield and zinc content of maize grains. Pak. J. Agric. Sci. 2020, 57, 123–130. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Shivay, Y.S.; Kumar, D.; Prasad, R. Effect of zinc-enriched urea on productivity, zinc uptake and efficiency of an aromatic rice-wheat cropping system. Nutr. Cycl. Agroecosyst. 2008, 81, 229. [Google Scholar] [CrossRef]
- Moran, K. Micronutrient Product Types and Their Development; Proceedings, No. 545; International Fertiliser Society: York, UK, 2004; pp. 1–24. [Google Scholar]
- Mumtaz, M.Z.; Barry, K.M.; Baker, A.L.; Nichols, D.S.; Ahmad, M.; Zahir, Z.A.; Britz, M.L. Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: A possible mechanism for Zn solubilization. Rhizosphere 2019, 12, 100170. [Google Scholar] [CrossRef]
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 2020, 10, 36. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Saqib, M.; Abbas, G.; Akhtar, J.; Qamar, Z. Genotypic variation in rice for grain yield and quality as affected by salt-affected field conditions. J. Plant Nutr. 2018, 41, 233–242. [Google Scholar] [CrossRef]
- Ilyas, N.; Mazhar, R.; Yasmin, H.; Khan, W.; Iqbal, S.; Enshasy, H.E.; Dailin, D.J. Rhizobacteria isolated from saline soil induce systemic tolerance in wheat (Triticum aestivum L.) against salinity stress. Agronomy 2020, 10, 989. [Google Scholar] [CrossRef]
- Sohaib, M.; Zahir, Z.A.; Khan, M.Y.; Ans, M.; Asghar, H.N.; Yasin, S.; Al-Barakah, F.N. Comparative evaluation of different carrier-based multi-strain bacterial formulations to mitigate the salt stress in wheat. Saudi J. Biol. Sci. 2020, 27, 777–787. [Google Scholar] [CrossRef]
- Nadeem, F.; Azhar, M.; Anwar-ul-Haq, M.; Sabir, M.; Samreen, T.; Tufail, A.; Awan, H.U.; Juan, W. Comparative response of two rice (Oryza sativa L.) cultivars to applied zinc and manganese for mitigation of salt stress. J. Soil Sci. Plant Nutr. 2020, 18. [Google Scholar] [CrossRef]
- Khande, R.; Sharma, S.K.; Ramesh, A.; Sharma, M.P. Zinc solubilizing Bacillus strains that modulate growth, yield and zinc biofortification of soybean and wheat. Rhizosphere 2017, 4, 126–138. [Google Scholar] [CrossRef]
- Graham, R.; Archer, J.S.; Hynes, S.C. Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil 1992, 146, 241–250. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Raza, S.; Zia, M.; Mustafa, A.; Xu, M.; Wang, Y. Evaluating organic materials coating on urea as potential nitrification inhibitors for enhanced nitrogen recovery and growth of maize (Zea mays). Int. J. Agric. Biol. 2019, 22, 1102–1108. [Google Scholar]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N.; Al-Whaibi, M.H.; Bahkali, A.H. Nitrogen in relation to photosynthetic capacity and accumulation of osmoprotectant and nutrients in Brassica genotypes grown under salt stress. Agric. Sci. China 2010, 9, 671–680. [Google Scholar] [CrossRef]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef]
- Saeed, Z.; Naveed, M.; Imran, M.; Bashir, M.A.; Sattar, A.; Mustafa, A.; Xu, M. Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS ONE 2019, 14, e0213016. [Google Scholar] [CrossRef] [Green Version]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núñez-Delgado, A.; Saeed, Q.; et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Azhar, S.Q.; Kamran, M.; Zahir, Z.A.; Núñez-Delgado, A. Burkholderia phytofrmans PsJN and tree twigs derived biochar together retrieved Pb induced growth, physiological and biochemical disturbances by minimizing its uptake and translocation in mung bean (Vigna radiata L.). J. Environ. Manag. 2020, 257, 109974. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Z.; Zhang, H.; Min, Z.H. Comparative effects of salt and alkali stress on antioxidant system in cotton (Gossypium Hirsutum L.) leaves. Open Chem. 2019, 17, 1352–1360. [Google Scholar] [CrossRef]
- Wakeel, A.; Steffens, D.; Schubert, S. Potassium substitution by sodium in sugar beet (Beta vulgaris) nutrition on K-fixing soils. J. Plant Nutr. Soil Sci. 2010, 173, 127–134. [Google Scholar] [CrossRef]
- Yamamoto, A.; Sawada, H.; Shim, I.S.; Usui, K.; Fujihara, S. Effect of salt stress on physiological response and leaf polyamine content in NERICA rice seedlings. Plant Soil Environ. 2011, 57, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Higbie, S.M.; Wang, F.; Stewart, J.M.; Sterling, T.M.; Lindemann, W.C.; Hughs, E.; Zhang, J. Physiological response to salt (NaCl) stress in selected cultivated tetraploid cottons. Int. J. Agron. 2010, 2010, 643475. [Google Scholar] [CrossRef] [Green Version]
- Baghbani, A.; Namdari, A.; Kadkhodaie, A. Effect of salinity and nitrogen supply on nitrogen fixation nodules and nitrogen, sodium and potassium concentration of alfalfa cultivars. J. Basic Appl. Sci. Res. 2012, 2, 9978–9984. [Google Scholar]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Carpici, E.B.; Celik, N.; Bayram, G. The effect of salt stress on the growth, biochemical parameters and mineral element content of some maize (Zea mays L.) cultivars. Afr. J. Biotech. 2010, 9, 6937–6942. [Google Scholar] [CrossRef]
- Brar, B.S.; Singh, K.; Dheri, G.S.; Balwinder-Kumar. Carbon sequestration and soil carbon pools in a rice-wheat cropping system: Effect of long-term use of inorganic fertilizers and organic manure. Soil Tillage Res. 2013, 128, 30–36. [Google Scholar] [CrossRef]
- Karimi, G.; Ghorbanli, M.; Heidari, H.; Khavarinejad, R.A.; Assareh, M.H. The effect of NaCl on growth, water relation, osmolytes and ion content in Kochia Prostrate. Biol. Plant. 2005, 49, 301–304. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, L.; Wang, Z.; Lei, T.; Yin, X. Phytoremediation of zinc-contaminated soil and zinc-biofortification for human nutrition. In Phytoremediation and Biofortification; Springer Briefs in Molecular Science; Yin, X., Yuan, L., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 33–57. [Google Scholar]
- Goteti, P.K.; Daniel, L.; Emmanuel, A.; Desai, S.; Hassan, M.; Shaik, A. Prospective Zinc Solubilising Bacteria for Enhanced Nutrient Uptake and Growth Promotion in Maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Naveed, M.; Ozturk, L.; Nawaz, A. Pseudomonas-aided zinc application improves the productivity and biofortification of bread wheat. Crop Past. Sci. 2018, 69, 659. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Moodie, C.; Smith, H.; McCreery, R. Laboratory Manual for Soil Fertility; Department of Agronomy, State College of Washington Pullman: Washington, DC, USA, 1959. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Constable and, Co. Ltd.: London, UK, 1962. [Google Scholar]
- Mumtaz, M.Z.; Saqib, M.; Abbas, G.; Akhtar, J.; Ul-Qamar, Z. Drought stress impairs grain yield and quality of rice genotypes by impaired photosynthetic attributes and K nutrition. Rice Sci. 2020, 27, 5–9. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinhem, Germany, 1983; pp. 273–286. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-transferase, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid). Anal Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Roth, E.F.; Gilbert, H.S. The pyrogallol assay for superoxide dismutase: Absence of a glutathione artifact. Anal. Biochem. 1984, 137, 50–53. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; Division of Agriculture Science, University of California: Riverside, CA, USA, 1961. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Cram 101 Textbook Outlines to Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic internet: New York, NY, USA, 2007. [Google Scholar]





| Treatments | Salinity Conditions | Plant Height (cm) | Root Length (cm) | Germination Rate (%) | No. of Tillers Plant−1 |
|---|---|---|---|---|---|
| Control | Normal | 65.4 ± 1.2 c | 22.0 ± 0.44 d | 74.3 ± 1.91 c | 3.3 ± 0.38 bcd |
| Saline | 59.3 ± 0.9 d | 19.3 ± 0.27 e | 65.7 ± 2.10 d | 2.3 ± 0.33 d | |
| ZnSO4 coated urea | Normal | 81.1 ± 1.1 a | 27.4 ± 0.61 a | 92.3 ± 2.45 a | 4.6 ± 0.37 a |
| Saline | 79.7 ± 1.2 a | 24.1 ± 0.24 b | 82.7 ± 2.24 b | 3.7 ± 0.37 abc | |
| BAZ-coated urea | Normal | 80.0 ± 0.8 a | 26.7 ± 0.38 a | 90.7 ± 2.35 a | 4.0 ± 0.58 ab |
| Saline | 71.3 ± 0.9 b | 23.5 ± 0.43 bc | 80.0 ± 3.07 bc | 3.0 ± 0.12 bcd | |
| Bacillus sp. strain AZ6 | Normal | 78.6 ± 0.3 a | 22.6 ± 0.47 cd | 80.0 ± 1.86 bc | 3.7 ± 0.21 abc |
| Saline | 68.6 ± 0.8 b | 19.8 ± 0.18 e | 72.3 ± 1.41 cd | 2.7 ± 0.32 cd |
| Treatments | Salinity Conditions | 1000-Grain Weight (g) | Spike Length (cm) | Spike Weight (g) | No. of Grains Spike−1 |
|---|---|---|---|---|---|
| Control | Normal | 38.63 ± 0.42 b | 8.17 ± 0.44 de | 12.7 ± 1.14 c | 35.7 ± 1.14 d |
| Saline | 33.83 ± 0.46 c | 7.33 ± 0.17 e | 10.5 ± 0.93 d | 30.0 ± 1.64 e | |
| ZnSO4 | Normal | 44.93 ± 1.44 a | 11.26 ± 0.44 a | 16.7 ± 1.05 a | 48.7 ± 2.32 a |
| coated urea | Saline | 40.06 ± 0.18 b | 9.50 ± 0.29 bc | 14.0 ± 1.21 b | 42.0 ± 1.87 b |
| BAZ-coated urea | Normal | 44.01 ± 1.34 a | 10.67 ± 0.17 ab | 15.7 ± 0.88 a | 47.3 ± 2.75 a |
| Saline | 39.73 ± 1.08 b | 9.01 ± 0.58 cd | 13.8 ± 0.75 b | 40.7 ± 2.36 bc | |
| Bacillus sp. strain AZ6 | Normal | 38.13 ± 0.71 b | 8.16 ± 0.60 de | 12.9 ± 1.01 c | 37.3 ± 1.95 cd |
| Saline | 32.83 ± 0.55 c | 7.50 ± 0.31 e | 10.9 ± 0.64 d | 31.3 ± 2.16 e |
| Treatments | Salinity Conditions | Ascorbate Peroxidase (nmol mint−1 g−1) | Glutathione Peroxidase (nmol mint−1 g−1) | Glutathione Transferase (µmol mint−1 mg−1) | Glutathione Reductase (nmol mint−1 mg−1) | Catalase (nmol mint−1 mg−1) | Superoxide Dismutase (nmol mint−1 mg−1) |
|---|---|---|---|---|---|---|---|
| Control | Normal | 31.2 ± 1.41 b | 50.0 ± 1.75 c | 197.0 ± 6.85 c | 20.7 ± 0.95 b | 9.88 ± 0.48 c | 134.0 ± 5.55 bc |
| Saline | 45.1 ± 1.78 a | 74.6 ± 1.52 a | 297.0 ± 9.34 a | 30.3 ± 1.23 a | 15.4 ± 0.52 a | 189.0 ± 6.91 a | |
| ZnSO4 coated urea | Normal | 12.8 ± 1.06 d | 19.9 ± 1.38 e | 63.0 ± 4.44 d | 7.5 ± 0.73 c | 3.68 ± 0.35 d | 55.0 ± 4.43 f |
| Saline | 22.2 ± 1.24 c | 35.5 ± 1.72 d | 136.0 ± 4.14 d | 16.6 ± 0.77 b | 8.0 ± 0.40 c | 98.0 ± 4.65 de | |
| BAZ-coated urea | Normal | 14.7 ± 0.71 d | 26.4 ± 1.34 e | 105.0 ± 5.02 d | 9.5 ± 0.87 c | 4.67 ± 0.36 d | 75 ± 5.10 ef |
| Saline | 25.3 ± 1.43 bc | 43.1 ± 1.58 cd | 175.0 ± 7.23 c | 19.0 ± 0.99 b | 8.6 ± 0.40 c | 115.0 ± 4.36 cd | |
| Bacillus sp. strain AZ6 | Normal | 29.4 ± 1.27 b | 46.1 ± 1.97 c | 177.0 ± 8.66 c | 18.7 ± 1.02 b | 7.92 ± 0.43 c | 119.0 ± 5.99 cd |
| Saline | 40.0 ± 1.59 a | 64.3 ± 1.89 b | 259.0 ± 8.86 b | 26.5 ± 1.07 a | 12.6 ± 0.47 b | 161.0 ± 6.33 b |
| Treatments | Salinity Conditions | Nitrogen in Straw (g kg−1) | Nitrogen in Grains (g kg−1) | Phosphorous in Straw (g kg−1) | Phosphorous in Grains (g kg−1) | Potassium in Straw (g kg−1) | Potassium in Grains (g kg−1) |
|---|---|---|---|---|---|---|---|
| Control | Normal | 15.2 ± 0.65 c | 11.0 ± 0.42 cd | 1.62 ± 0.06 cd | 0.83 ± 0.03 c | 12.3 ± 0.51 cd | 8.5 ± 0.31 c |
| Saline | 10.5± 0.38 e | 7.2 ± 0.36 f | 1.07 ± 0.05 f | 0.54 ± 0.02 e | 8.7 ± 0.45 e | 6.1 ± 0.30 e | |
| ZnSO4 coated urea | Normal | 20.5 ± 0.58 a | 15.8 ± 0.54 a | 2.29 ± 0.07 a | 1.19 ± 0.05 a | 17.7 ± 0.69 a | 12.7 ± 0.47 a |
| Saline | 14.6 ± 0.44 cd | 10.5 ± 0.41 cde | 1.57 ± 0.07 de | 0.84 ± 0.04 cd | 13.3 ± 0.68 bc | 9.1 ± 0.37 bc | |
| BAZ-coated urea | Normal | 19.4 ± 0.51 ab | 13.7 ± 0.37 ab | 2.07 ± 0.05 ab | 1.05 ± 0.04 ab | 15.8 ± 0.54 ab | 10.6 ± 0.43 b |
| Saline | 14.0 ± 0.38 cd | 9.8 ± 0.33 de | 1.45 ± 0.05 de | 0.74 ± 0.04 cd | 11.9 ± 0.59 cd | 8.0 ± 0.39 cd | |
| Bacillus sp. strain AZ6 | Normal | 17.5 ± 0.52 b | 12.5 ± 0.36 bc | 1.87 ± 0.07 bc | 0.89 ± 0.03 bc | 14.3 ± 0.47 bc | 9.1 ± 0.33 bc |
| Saline | 11.7 ± 0.48 de | 8.5 ± 0.31 ef | 1.29 ± 0.04 f | 0.66 ± 0.03 de | 10.0 ± 0.51 de | 6.7 ± 0.28 de |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ain, N.U.; Naveed, M.; Hussain, A.; Mumtaz, M.Z.; Rafique, M.; Bashir, M.A.; Alamri, S.; Siddiqui, M.H. Impact of Coating of Urea with Bacillus-Augmented Zinc Oxide on Wheat Grown under Salinity Stress. Plants 2020, 9, 1375. https://doi.org/10.3390/plants9101375
Ain NU, Naveed M, Hussain A, Mumtaz MZ, Rafique M, Bashir MA, Alamri S, Siddiqui MH. Impact of Coating of Urea with Bacillus-Augmented Zinc Oxide on Wheat Grown under Salinity Stress. Plants. 2020; 9(10):1375. https://doi.org/10.3390/plants9101375
Chicago/Turabian StyleAin, Noor Ul, Muhammad Naveed, Azhar Hussain, Muhammad Zahid Mumtaz, Munazza Rafique, Muhammad Asaad Bashir, Saud Alamri, and Manzer H. Siddiqui. 2020. "Impact of Coating of Urea with Bacillus-Augmented Zinc Oxide on Wheat Grown under Salinity Stress" Plants 9, no. 10: 1375. https://doi.org/10.3390/plants9101375
APA StyleAin, N. U., Naveed, M., Hussain, A., Mumtaz, M. Z., Rafique, M., Bashir, M. A., Alamri, S., & Siddiqui, M. H. (2020). Impact of Coating of Urea with Bacillus-Augmented Zinc Oxide on Wheat Grown under Salinity Stress. Plants, 9(10), 1375. https://doi.org/10.3390/plants9101375