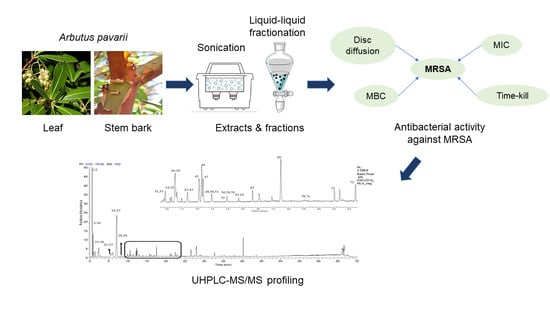
Antibacterial Activity of Arbutus pavarii Pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity Of A. pavarii Crude Extracts and Solvent Fractions
2.2. Bacteriostatic (MIC) and Bactericidal (MBC) Effects of Bioactive Extracts and Fractions
2.3. Time–Kill Curve for Ethyl Acetate Fraction of the Leaf
2.4. UHPLC-ESI–MS/MS Profile of the EtOAc fraction
2.4.1. Identification of Phenolic Acids and Derivatives
2.4.2. Identification of Flavan-3-ol and Derivatives
2.4.3. Identification of Flavonols and Derivatives
2.4.4. Identification of Other Compounds
3. Materials and Methods
3.1. Plant Materials, Extraction and Fractionation
3.2. Bacterial Strains and Preparation of Inoculum
3.3. Disc Diffusion Assay
3.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Values
3.5. Time–Kill Curve
3.6. UHPLC-ESI-MS/MS Analysis
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laken, I.I.; Musah Monday, D.M.; Mohammed, S.H.; Paiko, Y.B. Phytochemical and antibacterial activity of chrysanthellum indicum (linn) extracts. Afr. J. Environ. Nat. Sci. Res. 2019, 2, 73–82. [Google Scholar]
- Prabhoo, R.; Chaddha, R.; Iyer, R.; Mehra, A.; Ahdal, J.; Jain, R. Overview of methicillin resistant Staphylococcus aureus mediated bone and joint infections in India. Orthop. Rev. 2019, 11, 8070. [Google Scholar] [CrossRef] [Green Version]
- Haysom, L.; Cross, M.; Anastasas, R.; Moore, E.; Hampton, S. Prevalence and risk factors for methicillin-resistant Staphylococcus aureus (MRSA) infections in custodial populations: A systematic review. J. Correct. Health Care 2018, 24, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Fan, Y.L.; Zhao, F.; Ren, Q.C.; Wu, X.; Chang, L.; Gao, F. Quinolone derivatives and their activities against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2018, 157, 1081–1095. [Google Scholar] [CrossRef]
- Gonzalez-Bello, C. Antibiotic adjuvants–A strategy to unlock bacterial resistance to antibiotics. Bioorg. Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, e5. [Google Scholar] [CrossRef]
- Sahgal, G.; Sreeramanan, S.; Sasidharan, S.; Xavier, R.; Ong, M.T. Screening selected medicinal plants for antibacterial activity against Methicillin Resistant Staphylococcus aureus (MRSA). Adv. Nat. Appl. Sci. 2009, 3, 330–338. [Google Scholar]
- Oliveira, A.A.; Segovia, J.F.; Sousa, V.Y.; Mata, E.C.; Gonçalves, M.C.; Bezerra, R.M.; Junior, P.O.; Kanzaki, L.I. Antimicrobial activity of Amazonian medicinal plants. SpringerPlus 2013, 2, 371. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, A.; Boulos, L.; Kabiel, H.; Sharashy, O. Vegetation and species altitudinal distribution in Al-Jabal Al-Akhdar landscape, Libya. Pak. J. Bot. 2011, 43, 1885–1898. [Google Scholar]
- Alghazeer, R.; Abourghiba, T.; Ibrahim, A.; Zreba, E. Bioactive properties of some selected Libyan plants. J. Med. Plant Res. 2016, 10, 67–76. [Google Scholar]
- Tenuta, M.C.; Tundis, R.; Xiao, J.; Loizzo, M.R.; Dugay, A.; Deguin, B. Arbutus species (Ericaceae) as source of valuable bioactive products. Crit. Rev. Food Sci. 2019, 59, 864–881. [Google Scholar] [CrossRef]
- Delfin, J.C.; Watanabe, M.; Tohge, T. Understanding the function and regulation of plant secondary metabolism through metabolomics approaches. Theor. Exp. Plant Phys. 2019, 31, 127–138. [Google Scholar] [CrossRef]
- Alsabri, S.G.; El-Basir, H.M.; Rmeli, N.B.; Mohamed, S.B.; Allafi, A.A.; Zetrini, A.A.; Salem, A.A.; Mohamed, S.S.; Gbaj, A.; El-Baseir, M. Phytochemical screening, antioxidant, antimicrobial and anti-proliferative activities study of Arbutus pavarii plant. J. Chem. Pharm. Res. 2013, 5, 2–36. [Google Scholar]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Qaralleh, H.N. Chemical composition and antibacterial activity of Origanum ramonense essential oil on the β-lactamase and extended-spectrum β-lactamase urinary tract isolates. Bangl. J. Pharmacol. 2018, 13, 280–286. [Google Scholar] [CrossRef]
- Oliveira Silva, E.; Batista, R. Ferulic acid and naturally occurring compounds bearing a feruloyl moiety: A review on their structures, occurrence, and potential health benefits. Compr. Rev. Food Sci. F. 2017, 16, 580–616. [Google Scholar] [CrossRef] [Green Version]
- Okwu, M.U.; Olley, M.; Akpoka, A.O.; Izevbuwa, O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Quantifying the bioactivity of plant extracts during screening and bioassay-guided fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institue: Wayne, PA, USA, 2012. [Google Scholar]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. 2009, 23, 791–815. [Google Scholar] [CrossRef] [Green Version]
- Ramli, S.; Radu, S.; Shaari, K.; Rukayadi, Y. Antibacterial activity of ethanolic extract of Syzygium polyanthum L. (Salam) leaf against foodborne pathogens and application as food sanitizer. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shibani, F.A. A Pharmacognostical Study of Arbutus Pavarii Pampan. Family Ericaceae and Sarcopoterium Spinosum L. Family Rosaceae Growing in Libya. Ph.D. Thesis, Cairo University, Giza, Egypt, 2017. [Google Scholar]
- Hasan, H.H.; Habib, I.H.; Gonaid, M.H.; Islam, M. Comparative phytochemical and antimicrobial investigation of some plants growing in Al Jabal Al-Akhdar. J. Nat. Prod. Plant Resour. 2011, 1, 15–23. [Google Scholar]
- Kumar, S.; Chandra, P.; Bajpai, V.; Singh, A.; Srivastava, M.; Mishra, D.K.; Kumar, B. Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind. Crops. Prod. 2015, 69, 143–152. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Seeram, N.P. Liquid chromatography coupled with time-of-flight tandem mass spectrometry for comprehensive phenolic characterization of pomegranate fruit and flower extracts used as ingredients in botanical dietary supplements. J. Sep. Sci. 2018, 41, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Karar, M.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaf, fruits and their herbal derived drops (Crataegutt Tropfen). J. Chem. Bio. Therap. 2015, 1, 102. [Google Scholar]
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Characterization of phenolic composition in chestnut leaf and beer residue by LC-DAD-ESI-MS. LWT-Food Sci. Technol. 2016, 68, 52–58. [Google Scholar] [CrossRef]
- Singh, A.P.; Wang, Y.; Olson, R.M.; Luthria, D.; Banuelos, G.S.; Pasakdee, S.; Vorsa, N.; Wilson, T. LC-MS-MS analysis and the antioxidant activity of flavonoids from eggplant skins grown in organic and conventional environments. J. Food Sci. Nutr. 2017, 8, 869. [Google Scholar] [CrossRef] [Green Version]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef]
- Stöggl, W.M.; Huck, C.W.; Bonn, G.K. Structural elucidation of catechin and epicatechin in sorrel leaf extracts using liquid-chromatography coupled to diode array-, fluorescence-, and mass spectrometric detection. J. Sep. Sci. 2004, 27, 524–528. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Han, G.; Zou, L.; Lv, L.; Zhou, Q.; Li, N. LC MS/MS for simultaneous determination of four major active catechins of tea polyphenols in rat plasma and its application to pharmacokinetics. Chin. Herb. Med. 2010, 2, 289–296. [Google Scholar]
- Qing, L.S.; Xue, Y.; Zhang, J.G.; Zhang, Z.F.; Liang, J.; Jiang, Y.; Liu, Y.M.; Liao, X. Identification of flavonoid glycosides in Rosa chinensis flowers by liquid chromatography–tandem mass spectrometry in combination with 13C nuclear magnetic resonance. J. Chromatogr. A 2012, 1249, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.Z.; Wu, W.; Jiao, L.L.; Yang, P.F.; Guo, M.Q. Analysis of flavonoids in lotus (Nelumbo nucifera) leaf and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
- De la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; Sato, Y.; Murakami, K.; Takaishi, Y.; Arakaki, N.; Higuti, T. Alkyl gallates, intensifiers of β-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the antibacterial activity of 3-O-octanoyl-(–)-epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef]
- Lambert, P.A.; Hammond, S.M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 1973, 54, 796–799. [Google Scholar]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Shiota, S.; Shimizu, M.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked reduction in the minimum inhibitory concentration (MIC) of β-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis). Biol. Pharm. Bull. 1999, 22, 1388–1390. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Ma, L.; Wen, Y.; Wang, H.; Zhang, S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules 2014, 19, 12630–12639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhadrami, H.A.; Hamed, A.A.; Hassan, H.M.; Belbahri, L.; Rateb, M.E.; Sayed, A.M. Flavonoids as Potential anti-MRSA Agents through Modulation of PBP2a: A Computational and Experimental Study. Antibiotics 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial mechanism of hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Lee, K.; Han, S.; Yong, D.; Hwang, J.K. In vitro activities of panduratin A against clinical Staphylococcus strains. AAC 2009, 53, 4529–4532. [Google Scholar] [CrossRef] [Green Version]
- Alsohaili, S.A.; Al-fawwaz, A.T. Composition and antimicrobial activity of Achillea fragrantissima essential oil using food model media. Eur. Sci. J. 2014, 10, 156–165. [Google Scholar]


| MRSA Strains | CHX | CH3OH | EtOAc | n-BuOH | |||
|---|---|---|---|---|---|---|---|
| IZD | % RIZD | IZD | % RIZD | IZD | % RIZD | ||
| Leaf | |||||||
| ATCC 700699 | 8.00 ± 0.00 | 9.33 ± 0.57 | 120.83 ± 7.22 | 13.67 ± 0.57 | 170.83 ± 7.22 | 8.33 ± 0.57 | 100.00 ± 5.59 |
| KCCM 12255 | 7.00 ± 0.00 | 8.00 ± 0.00 | 114.29 ± 0.00 | 12.00 ± 0.00 | 171.43 ± 0.00 | 7.00 ± 0.00 | 100.00 ± 0.00 |
| MRSA1 | 10.33 ± 0.57 | 9.67 ± 0.57 | 93.58 ± 5.59 | 13.67 ± 1.15 | 132.30 ± 5.59 | n.a | n.d |
| MRSA2 | 10.33 ± 0.57 | 9.33 ± 0.57 | 83.90 ± 5.59 | 13.00 ± 0.00 | 125.85 ± 0.00 | n.a | n.d |
| Stem bark | |||||||
| ATCC 700699 | 8.00 ± 0.00 | 10.00 ± 0.00 | 125.00 ± 0.00 | 9.00 ± 0.00 | 112.50 ± 0.00 | 9.00 ± 0.00 | 112.50 ± 0.00 |
| KCCM 12255 | 7.00 ± 0.00 | 7.00 ± 0.00 | 100.00 ± 0.00 | 8.00 ± 0.00 | 114.29 ± 0.00 | 7.00 ± 0.00 | 100.00 ± 0.00 |
| MRSA1 | 10.33 ± 0.57 | 9.00 ± 0.00 | 87.12 ± 0.00 | 9.00 ± 0.00 | 87.12 ± 0.00 | n.a | n.d |
| MRSA2 | 10.33 ± 0.57 | 7.67 ± 0.57 | 74.22 ± 5.59 | 8.33 ± 0.57 | 80.67 ± 5.59 | 7.33 ± 0.57 | 70.99 ± 5.59 |
| MRSA Strains | Parts | CH3OH | EtOAc | n-BuOH | CHX | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| ATCC 700699 | Leaf | 0.08 | 0.16 | 0.08 | 0.16 | 0.04 | 0.08 | 0.02 | 0.63 |
| Stem bark | 0.63 | 1.25 | 1.25 | 2.50 | 2.50 | 5.00 | |||
| KCCM 12255 | Leaf | 0.63 | 1.25 | 0.31 | 1.25 | 0.63 | 1.25 | 0.02 | 0.63 |
| Stem bark | 1.25 | 2.50 | 1.25 | 2.50 | 2.50 | 5.00 | |||
| MRSA1 | Leaf | 1.25 | 2.50 | 1.25 | 2.50 | 2.50 | 5.00 | 0.03 | 0.13 |
| Stem bark | 1.25 | 2.50 | 1.25 | 2.50 | 2.50 | 5.00 | |||
| MRSA2 | Leaf | 1.25 | 2.50 | 1.25 | 2.50 | 1.25 | 2.50 | 0.03 | 0.13 |
| Stem bark | 1.25 | 2.50 | 0.63 | 1.25 | 0.63 | 1.25 | |||
| MRSA Strains | Total Activity in (mL/g) | |||||
|---|---|---|---|---|---|---|
| Leaf | Stem Bark | |||||
| CH3OH | EtOAc | n-BuOH | CH3OH | EtOAc | n-BuOH | |
| ATCC 700699 | 4007.6 | 1078.1 | 2235.89 | 452.35 | 61.18 | 54.22 |
| KCCM 12255 | 500.8 | 269 | 139.52 | 226.16 | 61.18 | 54.22 |
| MRSA1 | 250.4 | 67.36 | 34.88 | 113.08 | 61.18 | 54.22 |
| MRSA2 | 62.6 | 2158.97 | 69.76 | 226.16 | 122.37 | 216.90 |
| No | Retention Time (Rt) (min) | [M-H]− (m/z) | MS/MS Fragment Ions (m/z) | Compound Identity | Molecular Formula |
|---|---|---|---|---|---|
| Phenolic Acids and Derivatives | |||||
| 2 | 0.78 | 331.0668 | 271.05, 211.02, 169.01 | Gallic acid hexoside I | C13H16O10 |
| 4 | 1.04 | 331.0669 | 271.05, 211.02, 169.01 | Gallic acid hexoside II | C13H16O10 |
| 5 | 1.18 | 169.0131 | 125.02 | Gallic acid | C7H6O5 |
| 6 | 1.22 | 331.0668 | 271.05, 211.02, 169.01 | Gallic acid hexoside III | C13H16O10 |
| 7 | 1.23 | 343.0668 | 191.06, 169.01, 125.02 | Galloylquinic acid | C14H16O10 |
| 8 | 1.73 | 315.0720 | 153.02, 152.01, 109.03, 108.02 | Dihydroxybenzoic acid-O-hexoside | C13H16O9 |
| 11 | 2.89 | 483.0774 | 439.09, 424.54, 331.07, 313.06, 287.08, 271.05, 211.02, 169.01 | Di-O-galloylhexose | C20H20O14 |
| 14 | 3.43 | 329.0878 | 167.03, 152.01, 123.04, 108.02 | Vanillic acid-O-hexoside | C14H18O9 |
| 16 | 3.95 | 635.0888 | 465.07, 313.06, 271.05, 211.02, 169.01 | Tri-O-galloylhexose | C27H24O18 |
| Flavan-3-ol and Derivatives | |||||
| 9 | 1.83 | 305.06638 | 261.08, 179.03, 138.03, 137.02, 125.02 | (Epi)gallocatechin | C15H14O7 |
| 10 | 2.11 | 451.1254 | 289.07, 245.08, 151.04, 125.02 | (Epi)catechin-3-O-hexoside | C21H24O11 |
| 12 | 2.90 | 577.1334 | 451.10, 425.09, 407.08, 289.07, 287.06, 245.08, 125.02 | (Epi)catechin +(epi)catechin I | C30H26O12 |
| 13 | 3.11 | 289.0714 | 271.06, 245.08, 179.03, 165.02, 150.03, 137.02, 125.02 | Catechin | C15H14O6 |
| 15 | 3.88 | 289.0717 | 271.06, 245.08, 179.03, 165.02, 150.03, 137.02, 125.02 | Epicatechin | C15H14O6 |
| 17 | 4.03 | 729.1458 | 577.14, 559.13, 451.10, 425.09, 407.08, 289.07, 125.02 | (Epi)catechin gallate + (epi)catechin I | C37H30O16 |
| 20 | 5.20 | 441.0823 | 289.07, 245.08, 203.07, 169.01 | (Epi)catecin gallate | C22H18O10 |
| 21 | 5.25 | 729.1453 | 577.11, 407.08, 425.09, 289.07, 125.02 | (Epi)catechin gallate + (epi)catechin II | C37H30O16 |
| Flavonols and Derivatives | |||||
| 18 | 4.72 | 615.0989 | 463.09, 300.03, 301.03, 271.02, 179.00, 151.00, 169.01 | Quercetin-O-galloylhexoside | C28H24O16 |
| 19 | 4.98 | 609.1463 | 301.03, 300.03, 271.02, 255.03 | Quercetin-3-O-deoxyhexosyl-hexoside | C27H30O16 |
| 22 | 5.40 | 463.0884 | 317.03, 316.02, 287.02, 271.02, 179.00, 151.00 | Myricetin-3-O-deoxyhexoside | C21H20O12 |
| 23 | 5.55 | 433.0775 | 301.03, 300.03, 271.02, 255.03 | Quercetin-3-O-pentoside | C20H18O11 |
| 24 | 5.56 | 447.0931 | 285.04, 284.03, 255.03, 227.03 | kaempferol-3-O-hexoside | C2120O11 |
| 25 | 5.99 | 447.0931 | 301.03, 300.03, 271.02, 255.03 | Quercetin-3-O-deoxyhexoside | C21H20O11 |
| 26 | 6.30 | 463.0885 | 301.03, 300.03, 271.07, 255.03 | Quercetin-3-O-hexoside | C21H20O12 |
| 27 | 6.42 | 583.1099 | 463.09, 301.03, 300.03, 271.03, 255.03 | Quercetin-O-(p-hydroxy) benzonylhexoside | C28H24O14 |
| 28 | 7.53 | 301.0354 | 271.02, 255.03, 179.00, 151.00, 149.02, 121.03, 121.03, 107.01 | Quercetin | C15H10O7 |
| Others | |||||
| 1 | 0.76 | 191.0555 | 171.03, 127.04, 109.03, 93.03 | Quinic acid | C7H12O6 |
| 3 | 0.80 | 271.0453 | 211.02, 108.02 | Arbutin | C12H16O7 |
| Plant Part | Solvent | Weight (g) | Yield % | Physical Appearance |
|---|---|---|---|---|
| Leaf | CH3OH | 470.00 | 31.13 | Dark greenish brown gum |
| Hex | 18.60 | 3.95 | Dark green gum | |
| CHCl3 | 24.90 | 5.29 | Green gum | |
| EtOAc | 126.48 | 26.91 | Dark orange gum | |
| n-BuOH | 131.00 | 27.87 | Brown gum | |
| Stem Bark | CH3OH | 141.35 | 28.27 | Greenish brown gum |
| Hex | 11.42 | 8.08 | Dark green gum | |
| CHCl3 | 3.65 | 2.58 | Green gum | |
| EtOAc | 38.24 | 27.05 | Dark brown gum | |
| n-BuOH | 67.78 | 47.95 | Dark brown gum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzgaia, N.; Awin, T.; Elabbar, F.; Abdusalam, K.; Lee, S.Y.; Rukayadi, Y.; Abas, F.; Shaari, K. Antibacterial Activity of Arbutus pavarii Pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction. Plants 2020, 9, 1539. https://doi.org/10.3390/plants9111539
Buzgaia N, Awin T, Elabbar F, Abdusalam K, Lee SY, Rukayadi Y, Abas F, Shaari K. Antibacterial Activity of Arbutus pavarii Pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction. Plants. 2020; 9(11):1539. https://doi.org/10.3390/plants9111539
Chicago/Turabian StyleBuzgaia, Nawal, Tahani Awin, Fakhri Elabbar, Khaled Abdusalam, Soo Yee Lee, Yaya Rukayadi, Faridah Abas, and Khozirah Shaari. 2020. "Antibacterial Activity of Arbutus pavarii Pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction" Plants 9, no. 11: 1539. https://doi.org/10.3390/plants9111539
APA StyleBuzgaia, N., Awin, T., Elabbar, F., Abdusalam, K., Lee, S. Y., Rukayadi, Y., Abas, F., & Shaari, K. (2020). Antibacterial Activity of Arbutus pavarii Pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction. Plants, 9(11), 1539. https://doi.org/10.3390/plants9111539