Prostanthera (Lamiaceae) as a ‘Cradle of Incense’: Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian ‘Mint Bush’ Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Sample Preparation and Hydrodistillation
2.3. Chromatography Methods and Compound Identification (GC–MS and NMR Analysis)
2.4. Multivariate Analysis
3. Results and Discussion
3.1. The History of Essential Oil Studies in Prostanthera
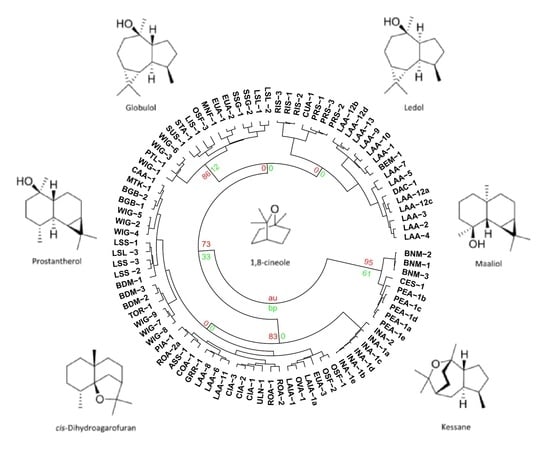
3.2. The Multivariate Analysis
3.3. Taxa Affiliated with Prostanthera Ovalifolia
3.4. Taxa Afiliated with Prostanthera Rotundifolia
3.5. Chemical Groups in Prostanthera
3.6. The Philosophy of Chemophenetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Althofer, G.W. Cradle of Incense: The Story of Australian Prostanthera; Private Publication: Sydney, Australia, 1978. [Google Scholar]
- Tang, K.S.C.; Konczak, I.; Zhao, J. Phenolic compounds of the Australian native herb prostanthera rotundifolia and their biological activities. Food Chem. 2017, 233, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; Methuen Australia Pty Ltd.: North Rhyde, Australia, 2011. [Google Scholar]
- Barr, A. Traditional Bush Medicines: An Aboriginal Pharmacopoeia; Greenhouse Publications Pty Ltd.: Richmond, VIC, Australia, 1988. [Google Scholar]
- Sadgrove, N.J.; Lyddiard, D.; Collins, T.L.; Greatrex, B.W.; Jones, G.L. Genifuranal and other derivatives: Smoking desert plants. Acta Hortic. 2016, 1125, 181–188. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Jones, G.L. Reviewing the importance of aromatic medicinal plants in the traditional pharmacopoeia of australian aboriginal people. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014); Acta Horticulturae: Leuven, Belgium, 2014; Volume 1125, pp. 297–302. [Google Scholar]
- Palá-Paúl, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Essential oil composition of two variants of Prostanthera lasianthos labill. From Australia. Biochem. Syst. Ecol. 2006, 34, 48–55. [Google Scholar] [CrossRef]
- Sadgrove, N.J. Comparing essential oils from australia’s ‘Victorian Christmas Bush’ (Prostanthera lasianthos labill., Lamiaceae) to closely allied new species: Phenotypic plasticity and taxonomic variability. Phytochemistry 2020, 176, 112403. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Jones, G.L. A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.L.; Sadgrove, N.J. Ethnopharmacology in Australia and Oceania. In Ethnopharmacology—A Reader; Heinrich, M., Jäger, A.K., Eds.; John Wiley & Sons: West Sussex, UK, 2015. [Google Scholar]
- ThePlantList. The Plant List. Available online: http://www.theplantlist.org/ (accessed on 26 August 2020).
- AVH. Australasian Virtual Herbarium. Available online: https://avh.chah.org.au/ (accessed on 25 March 2020).
- Collins, T.L.; Jones, G.L.; Sadgrove, N. Volatiles from the rare Australian desert plant Prostanthera centralis b.J.Conn (Lamiaceae): Chemical composition and antimicrobial activity. Agriculture 2014, 4, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Sadgrove, N.J.; Greatrex, B.W.; Jones, G.L. A-cyclodextrin encapsulation enhances antimicrobial activity of cineole-rich essential oils from Australian species of Prostanthera (Lamiaceae). Nat. Volatiles Essent. OIls 2015, 2, 30–38. [Google Scholar]
- NIST. Nist Chemistry Webbook: Nist Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 25 March 2020).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Southwell, I.A.; Tucker, D.J. Cis-dihydroagarofuran from Prostanthera sp. aff. ovalifolia. Phytochemistry 1993, 22, 857–862. [Google Scholar] [CrossRef]
- Dellar, J.E.; Cole, M.D.; Gray, A.I.; Gibbons, S.; Waterman, P.G. Antimicrobial sesquiterpenes from Prostanthera aff. melissifolia and P. rotundifolia. Phytochemistry 1994, 36, 957–960. [Google Scholar] [CrossRef]
- Kaplan, M.A.C.; Pugialli, H.R.L.; Lopes, D.; Gottlieb, H.E. The stereochemistry of ledol from Renealmia chrysotrycha: An nmr study. Phytochemistry 2000, 55, 749–753. [Google Scholar] [CrossRef]
- Toyota, M.; Tanaka, M.; Asakawa, Y. A revision of the 13C NMR spectral assignment of globulol. Spectroscopy 1999, 14, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Gijsen, H.J.M.; Wijnberg, J.B.P.A.; van Ravenswaay, C.; de Groot, A. Rearrangement reactions of aromadendrane derivatives. The synthesis of (+)-maaliol, starting from natural (+)-aromadendrene-iv. Tetrahedron 1994, 50, 4733–4744. [Google Scholar] [CrossRef]
- Suzuki, R.; Shimodaira, H. Pvclust: An r package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Padilla-González, G.F.; Aldana, J.A.; Costa, F.B.D. Chemical characterization of two morphologically related Espeletia (Asteraceae) species and chemometric analysis based on essential oil components. Rev. Bras. Farmacogn. 2016, 26, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Padilla-González, G.F.; Frey, M.; Gómez-Zeledón, J.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacón (Smallanthus sonchifolius, Asteraceae). Sci. Rep. 2019, 9, 13178. [Google Scholar] [CrossRef]
- Lassak, E.V. New essential oils from the australian flora (october 1980)—Perfumes and flavours symphony of nature. In 8th International Congress of Essential Oils, Fedarom Grasse, France—Paper No. 120; Fedarom Grasse: Grasse, France, 1980; pp. 409–415. [Google Scholar]
- Lassak, E.V.; Southwell, I.A. Essential oil isolations from the Australian flora. Int. Flavours Food Addit. 1977, 8, 126–132. [Google Scholar]
- Conn, B.J. A taxonomic revision of Prostanthera labill. Section klanderia (f.V. Muell.) benth. (labiatae). J. Adel. Bot. Gard. 1984, 6, 207–348. [Google Scholar]
- Conn, B.J.; Henwood, M.J.; Proft, K.M.; Wilson, T.C. Molecular phylogenetics reveals a new species of prostanthera from tropical queensland with links to more southerly taxa. Telopea 2016, 19, 13–22. [Google Scholar]
- Wilson, T.C.; Conn, B.J.; Henwood, M.J. Molecular phylogeny and systematics of Prostanthera (lamiaceae). Aust. Syst. Bot. 2012, 25, 341–352. [Google Scholar] [CrossRef]
- Baker, R.T.; Smith, H.G. On a new species of Prostanthera and its essential oil. J. Proc. R. Soc. NSW 1912, 46, 103–110. [Google Scholar]
- McKern, H.H.G. Arthur de ramon penfold. J. Proc. R. Soc. NSW 1980, 113, 100. [Google Scholar]
- McKern, H.H.G. Arthur de ramon penfold, 1890–1980. Chem. Aust. 1981, 48, 327. [Google Scholar]
- Hellyer, R.O. Occurence of maaliol, elemol, and globulol in some australian essential oils. Aust. J. Chem. 1962, 15, 157. [Google Scholar] [CrossRef] [Green Version]
- Nararak, J.; Sathantriphop, S.; Kongmee, M.; Mahiou-Leddet, V.; Ollivier, E.; Manguin, S.; Chareonviriyaphap, T. Excito-repellent activity of β-caryophyllene oxide against Aedes aegypti and Anopheles minimus. Acta Trop. 2019, 197, 105030. [Google Scholar] [CrossRef] [PubMed]
- Penfold, A.R. The essential oil of Eriostemon myoporoides (de candolle). J. Proc. R. Soc. NSW 1925, 59, 206–211. [Google Scholar]
- Buchi, G.; Wittenau, S.V.; White, D.M. Terpenes. X. The constitution of maaliol. J. Am. Chem. Soc. 1959, 81, 1968–1980. [Google Scholar] [CrossRef]
- Raina, A.P.; Negi, K.S. Essential oil composition of Valeriana jatamansi jones from himalayan regions of India. Indian J. Pharm. Sci. 2015, 77, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Valeriana wallichii dc (maaliol chemotype): Antinociceptive studies on experimental animal models and possible mechanism of action. Pharmacologia 2012, 3, 432–437. [Google Scholar] [CrossRef]
- Sadgrove, N.; Telford, I.R.H.; Greatrex, B.W.; Dowell, A.; Jones, G.L. Dihydrotagetone, an unusual fruity ketone, is found in enantiopure and enantioenriched forms in additional australian native taxa of Phebalium (Rutaceae: Boronieae). Nat. Prod. Commun. 2013, 8, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Padilla-González, G.F.; Diazgranados, M.; Oliveira, T.B.; Chagas-Paula, D.; Da Costa, F.B. Chemistry of the subtribe Espeletiinae (Asteraceae) and its correlation with phylogenetic data: An in silico chemosystematic approach. Bot. J. Linn. Soc. 2018, 186, 18–46. [Google Scholar] [CrossRef]
- Zidorn, C. Plant chemophenetics—A new term for plant chemosystematics/plant chemotaxonomy in the macro-molecular era. Phytochemistry 2019, 163, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Telford, I.R.H.; Padilla-González, G.F.; Greatrex, B.W.; Bruhl, J.J. GC–MS ‘chemophenetics’ on Australian pink-flowered Phebalium (Rutaceae) using herbarium leaf material demonstrates phenetic agreement with putative new species. Phytochem. Lett. 2020, 38, 112–120. [Google Scholar] [CrossRef]
- De Queiroz, K. Species concepts and species delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadgrove, N.J.; Jones, G.L. Cytogeography of essential oil chemotypes of Eremophila longifolia f. Muell (Schrophulariaceae). Phytochemistry 2014, 105, 43–51. [Google Scholar] [CrossRef]






| - | - | Taxon | LAA-1 | LAA-2 | LAA-3 | LAA-4 | LAA-5 | LAA-6 | LAA-7 | LAA-8 | LAA-9 | LAA-10 | LAA-11 | LAA-12a | LAA-12b | LAA-12c | LAA-12d | LAA-13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | Yield * | 1.4 | 0.9 | 1.6 | 1.3 | 1.1 | 1.4 | 0.6 | 1.4 | 0.5 | 1.2 | 1.1 | 1.2 | 1.9 | 1.4 | 2.0 | 1.5 |
| Compound Name | RI-a ** | RI-b ** | ||||||||||||||||
| α-Thujene | 928 | 924 | - | 0.8 | 1.6 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| α-Pinene | 934 | 932 | - | 0.8 | 2.0 | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| Sabinene | 973 | 969 | 1.1 | 0.8 | 1.8 | 0.7 | 0.4 | - | - | 1.1 | - | - | - | 0.6 | 0.8 | 0.7 | 0.8 | - |
| β-Pinene | 979 | 974 | 1.0 | 2.1 | 5.8 | 1.6 | 0.6 | - | - | 1.0 | - | - | - | 0.4 | 0.6 | 0.5 | 0.7 | 0.7 |
| α-Phellandrene | 1005 | 1002 | - | 0.4 | 0.4 | 0.3 | 2.1 | 0.8 | - | - | - | - | - | 1.0 | 0.7 | 0.8 | 1.1 | 1.3 |
| p-Cymene | 1025 | 1020 | 2.5 | 1.5 | - | 2.8 | 6.2 | 12.1 | 1.7 | 8.8 | 2.8 | 1.7 | 5.8 | 2.2 | 1.9 | 3.8 | 3.2 | 11.2 |
| Limonene | 1029 | 1024 | - | - | 0.8 | 0.5 | 0.7 | - | - | - | - | 0.7 | - | 0.5 | - | 0.5 | - | 3.3 |
| 1,8-Cineole | 1034 | 1031 | 27.7 | 20.5 | 21.4 | 21.9 | 21.5 | 53.6 | 17.0 | 65.9 | 13.8 | 17.7 | 43.9 | 24.3 | 28.7 | 28.7 | 41.0 | 28.8 |
| Linalool | 1101 | 1100 | - | 0.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| E-Thujone | 1115 | 1112 | - | - | - | 0.8 | - | 0.7 | - | - | - | - | - | - | - | - | - | - |
| E-Pinocarveol | 1136 | 1135 | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 | - | 0.5 | - |
| Santalone | 1179 | 1177 | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - |
| Terpinen-4-ol | 1180 | 1174 | - | 0.3 | - | 0.4 | - | 0.7 | - | - | - | - | 0.5 | 0.3 | 0.4 | 0.4 | 0.5 | - |
| α-Terpineol | 1192 | 1186 | 1.9 | 1.2 | 2.4 | 0.4 | 1.2 | - | - | - | - | - | 3.9 | 0.9 | - | 1.0 | 1.7 | - |
| Myrtenal | 1201 | 1195 | - | 0.7 | 0.6 | 1.2 | - | - | - | - | - | - | 0.5 | 0.4 | 0.5 | 0.4 | 0.4 | - |
| Terpinyl acetate | 1328 | 1316 | - | - | - | - | - | - | - | - | - | - | - | - | 0.6 | - | - | - |
| α-terpineol acetate | 1349 | 1344 | - | - | - | 1.8 | 0.7 | - | - | - | - | - | - | - | 4.0 | 1.1 | - | - |
| E-Caryophyllene | 1419 | 1417 | - | - | - | - | 0.8 | 0.6 | - | - | - | - | 2.0 | - | - | - | - | - |
| Dehydroaromadendrane | 1466 | 1460 | - | - | - | - | - | - | - | - | - | - | - | 0.5 | 1.1 | 0.5 | - | - |
| α-Amorphene | 1481 | 1485 | - | - | - | - | - | - | - | - | - | - | - | 1.0 | 1.1 | 1.0 | - | - |
| Z-β-Guaiene | 1491 | 1492 | - | - | 0.6 | - | - | - | - | - | - | - | 1.9 | - | - | - | - | 1.6 |
| Bicyclogermacrene | 1504 | 1500 | 0.9 | - | - | - | 10.8 | 4.5 | - | 0.9 | - | - | 10.7 | 0.3 | 0.8 | - | - | - |
| Z-Dihydroagarofuran | 1525 | 1519 | 59.4 | 14.9 | - | 18.9 | - | 19.0 | - | 16.5 | 59.2 | 42.1 | 21.5 | 2.8 | 9.6 | - | 23.4 | 28.4 |
| δ-Cadinene | 1527 | 1522 | - | 0.7 | - | 0.3 | - | - | - | - | - | - | - | 0.5 | - | - | - | - |
| Kessane | 1532 | 1529 | - | 24.4 | 43.7 | 28.8 | 37.8 | - | 76.9 | - | 17.3 | 31.8 | - | 32.2 | 13.9 | 34.7 | 10.0 | 5.1 |
| α-Cadinene | 1535 | 1537 | - | 0.4 | - | 0.5 | 0.7 | 1.6 | - | - | - | - | - | 1.6 | 0.7 | 1.6 | 0.5 | - |
| Epiglobulol | 1573 | NMR | - | - | - | - | 2.0 | - | - | 2.9 | - | - | 0.4 | - | 6.8 | - | - | - |
| Spathulenol | 1578 | 1577 | - | - | - | - | - | 1.9 | - | - | - | - | 2.5 | - | - | - | - | - |
| Caryophyllene oxide | 1584 | 1582 | - | - | - | - | - | - | - | - | - | - | 1.4 | - | - | - | - | - |
| Globulol | 1590 | 1590 | - | 19.5 | 13.3 | 14.7 | - | 0.8 | - | - | - | 3.1 | 1.9 | 27.9 | 9.1 | 23.4 | 6.9 | 4.8 |
| Viridiflorol | 1601 | 1592 | - | - | - | 0.7 | - | - | - | - | - | - | - | - | - | - | - | - |
| Prostantherol | 1602 | NMR | 1.3 | 1.1 | - | - | 3.1 | 1.2 | 2.9 | - | 1.3 | 0.5 | 1.9 | 1.4 | 16.0 | - | 8.0 | 14.7 |
| Ledol | 1610 | 1602 | - | - | - | - | 3.3 | 2.6 | - | - | 5.5 | 2.2 | - | - | 1.2 | - | 0.7 | - |
| Cubenol | 1619 | 1618 | - | 9.1 | 5.4 | 2.7 | - | - | - | - | - | - | - | - | - | - | - | - |
| Alloaromadendrene epoxide | 1638 | 1632 | 0.7 | 0.3 | - | - | 1.4 | - | - | 2.8 | - | - | 1.1 | - | - | - | - | - |
| Cadinol-epi-alpha | 1648 | 1638 | - | - | - | - | - | - | - | - | - | - | - | 1.1 | 0.4 | 0.5 | 0.4 | - |
| α-Cadinol | 1661 | 1652 | - | - | - | - | - | - | - | - | - | - | - | 0.4 | - | 0.4 | - | - |
| n.d. | 1748 | n/a | 3.4 | - | - | - | 6.7 | - | 1.5 | - | - | - | - | - | 0.6 | - | - | - |
| - | - | Taxon | OVA-1 | CIA-1 | CIA-2 | CIA-3 | LAIA-1a | LAIA-1b | BNM-1 | BNM-2 | BNM-3 | BEM-1 | COA-1 | ROA-1 | ROA-2a | ROA-2b | ULN-1 | DAC-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | Yield * | 0.6 | 0.2 | 0.8 | 1.1 | 0.9 | 0.7 | 2.4 | 2.7 | 2.1 | 1.5 | 1.3 | 1.1 | 1.5 | 0.6 | 0.9 | 1.3 |
| Compound Name | RI-a ** | RI-b ** | ||||||||||||||||
| α-Thujene | 928 | 924 | 0.3 | - | 5.2 | - | - | - | - | - | - | - | 2.8 | - | - | - | - | - |
| α-Pinene | 934 | 932 | 11.6 | - | 0.8 | 0.7 | 5.5 | 13.9 | - | - | - | - | 1.5 | 0.7 | 0.4 | 0.5 | - | 0.4 |
| Camphene | 951 | 946 | 2.0 | - | - | - | 0.8 | 2.2 | - | - | - | - | - | - | - | - | - | - |
| Verbenene | 956 | 961 | 0.5 | - | - | - | 0.9 | 0.5 | - | - | - | - | - | - | - | - | - | - |
| Sabinene | 973 | 969 | - | - | 0.7 | - | 0.3 | 1.0 | - | - | - | - | 0.6 | 1.5 | 0.4 | 1.0 | - | 0.5 |
| β-Pinene | 979 | 974 | 1.4 | 0.5 | 1.1 | 1.5 | 0.3 | 1.5 | - | - | - | - | 1.5 | 0.9 | 0.5 | 0.8 | 0.8 | 0.6 |
| Myrcene | 987 | 988 | 0.4 | - | - | - | 0.3 | 0.4 | - | - | - | - | - | - | - | - | - | - |
| α-Phellandrene | 1005 | 1002 | 1.5 | - | 1.1 | - | 2.1 | 1.3 | 1.5 | 1.1 | 1.3 | - | - | 4.2 | 0.3 | 3.6 | - | 2.0 |
| p-Cymene | 1025 | 1020 | 6.2 | 12.7 | 17.5 | 19.3 | 7.6 | 4.2 | 3.4 | 2.6 | 3.0 | 1.2 | 7.2 | 4.5 | 3.1 | 4.7 | 2.0 | 9.5 |
| Limonene | 1029 | 1024 | 0.4 | - | - | 1.1 | 0.4 | 0.6 | - | - | - | - | 1.3 | - | - | 1.8 | - | 1.5 |
| 1,8-Cineole | 1034 | 1031 | 22.6 | 38.8 | 49.7 | 64.2 | 11.9 | 30.3 | 11.5 | 8.7 | 10.1 | 30.2 | 63.6 | 39.6 | 29.9 | 33.0 | 65.6 | 31.8 |
| p-Cymenene | 1091 | 1089 | - | - | - | - | 0.4 | 0.7 | - | - | - | - | - | - | - | - | - | - |
| Linalool | 1101 | 1100 | 0.2 | - | - | - | - | - | 0.8 | 0.6 | 0.7 | - | - | - | - | - | - | - |
| E-Thujone | 1115 | 1112 | 1.2 | 1.2 | 1.6 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| E-Pinocarveol | 1136 | 1135 | 1.0 | - | - | - | - | 1.0 | - | - | - | - | 0.9 | - | - | - | - | - |
| Z-Verbenol | 1143 | 1137 | - | - | - | - | 3.5 | - | - | - | - | - | - | - | 0.4 | - | - | - |
| E-Verbenol | 1147 | 1140 | - | - | - | - | 15.4 | 5.2 | - | - | - | - | - | - | - | - | - | - |
| Camphor | 1148 | 1150 | 5.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Nonadienal | 1152 | 1150 | 0.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Borneol | 1169 | 1170 | 8.4 | - | - | - | 4.1 | 5.2 | - | - | - | - | 0.9 | - | - | - | - | - |
| Santalone | 1179 | 1177 | - | - | - | - | - | 0.4 | - | - | - | - | 1.2 | - | - | - | - | - |
| Terpinen-4-ol | 1180 | 1174 | 0.4 | 0.4 | 0.9 | - | - | - | - | - | - | - | - | - | 0.3 | 0.4 | - | - |
| α-Terpineol | 1192 | 1186 | 3.7 | 0.4 | 1.3 | - | 1.0 | 2.7 | - | - | - | - | 1.8 | 0.4 | 0.9 | 0.7 | - | - |
| Myrtenal | 1201 | 1195 | 0.5 | 0.3 | - | - | 0.3 | 0.8 | - | - | - | - | 0.9 | - | - | - | - | - |
| Verbenone | 1213 | 1204 | 3.8 | - | - | - | 0.5 | 3.2 | - | - | - | - | - | - | - | - | - | - |
| Linalyl acetate | 1256 | 1254 | 0.2 | - | - | - | - | - | 1.5 | 1.2 | 1.4 | - | - | - | - | - | - | - |
| Bornyl acetate | 1287 | 1288 | 12.9 | - | - | - | 6.2 | 4.4 | - | - | - | - | - | - | - | - | - | - |
| Thymol | 1295 | 1289 | - | 0.5 | - | - | 0.5 | 0.4 | - | - | - | - | - | - | 0.3 | - | - | - |
| Terpinyl acetate | 1328 | 1316 | - | - | 0.9 | - | - | 0.6 | - | - | - | - | - | - | 0.3 | 0.6 | - | - |
| α-terpineol acetate | 1349 | 1344 | - | - | - | - | 0.5 | - | - | - | - | 0.6 | - | 3.1 | 1.4 | 4.6 | - | - |
| α-Copaene | 1373 | 1374 | 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| E-Caryophyllene | 1419 | 1417 | 0.4 | - | - | - | - | - | - | - | - | - | - | 2.3 | - | - | - | - |
| Aromandendrene | 1443 | 1439 | - | - | - | - | 0.3 | - | - | - | - | - | - | 0.4 | - | - | 0.8 | - |
| Alloaromadendrene | 1462 | 1458 | - | - | - | - | - | - | - | - | - | 0.7 | - | - | 2.0 | - | 1.3 | - |
| Dehydroaromadendrane | 1466 | 1460 | - | 0.3 | - | - | - | 1.3 | 2.1 | 1.6 | 1.9 | 0.9 | - | 4.6 | - | 1.3 | - | - |
| α-Amorphene | 1481 | 1485 | - | - | - | - | - | - | - | - | - | 2.1 | - | 2.6 | - | 2.6 | - | - |
| Z-β-Guaiene | 1491 | 1492 | - | - | - | - | - | - | - | - | - | 1.7 | - | - | 0.4 | 7.2 | 1.8 | - |
| Bicyclogermacrene | 1504 | 1500 | 3.5 | 2.3 | 1.4 | 0.8 | - | 2.6 | 2.0 | 1.5 | 1.8 | - | 0.8 | 1.9 | 0.4 | 1.7 | - | 1.3 |
| Z-Dihydroagarofuran | 1525 | 1519 | - | - | - | - | - | - | - | - | - | 48.8 | - | - | - | - | - | 1.2 |
| δ-Cadinene | 1527 | 1522 | - | - | - | - | - | 1.0 | - | - | - | - | - | - | - | - | - | - |
| Kessane | 1532 | 1529 | - | - | - | - | 16.7 | 5.7 | 2.5 | 1.9 | 2.2 | - | - | 11.0 | 3.6 | - | - | 8.3 |
| α-Cadinene | 1535 | 1537 | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - | - |
| Maaliol | 1566 | NMR | - | - | - | - | - | - | - | - | - | 0.9 | - | - | 0.7 | - | - | - |
| Epiglobulol | 1573 | NMR | 0.3 | - | - | - | - | - | - | - | - | - | - | - | 18.4 | - | 5.9 | - |
| Spathulenol | 1578 | 1577 | - | - | - | - | - | 0.3 | - | - | - | 0.5 | 2.7 | - | 0.4 | - | - | - |
| Caryophyllene oxide | 1584 | 1582 | 1.1 | 7.3 | 3.8 | 2.4 | - | - | - | - | - | 0.8 | - | - | - | - | 1.3 | 1.0 |
| Globulol | 1590 | 1590 | 0.7 | 1.2 | - | - | 20.2 | 5.6 | - | - | - | 7.2 | 12.1 | 2.3 | 29.5 | 0.8 | 1.8 | 41.2 |
| Viridiflorol | 1601 | 1592 | - | - | - | - | - | - | - | - | - | 1.7 | - | - | 2.3 | 0.7 | 1.5 | - |
| Prostantherol | 1602 | NMR | 2.9 | 27.0 | 13.8 | 9.9 | - | 0.7 | 49.0 | 61.2 | 55.1 | 1.1 | - | 3.2 | 0.4 | 28.9 | 6.8 | - |
| Ledol | 1610 | 1602 | 5.0 | 1.1 | - | - | - | 1.4 | 25.7 | 19.6 | 22.7 | - | - | 16.7 | 3.7 | 2.8 | - | - |
| Cubenol | 1619 | 1618 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.9 | - | - |
| Alloaromadendrene epoxide | 1638 | 1632 | - | 5.9 | - | - | - | - | - | - | - | - | - | - | - | - | 2.0 | - |
| Caryophylla-diene-ol | 1642 | 1644 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.6 |
| Cadinol-epi-alpha | 1648 | 1638 | - | - | - | - | - | - | - | - | - | 1.0 | - | - | - | - | - | - |
| α-Cadinol | 1661 | 1652 | - | - | - | - | - | - | - | - | - | 0.5 | - | - | - | - | - | - |
| n.d. | 1748 | n/a | - | - | - | - | - | 0.5 | - | - | - | - | - | - | - | - | 1.8 | - |
| Squamulosone | 1774 | NMR | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.4 | - | - |
| n.d. | 1818 | n/a | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.2 | - |
| n.d. | 1825 | n/a | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.2 | - |
| - | - | Taxon | LIS-1 | CUA-1 | PRS-1 | PRS-3 | PRS-2 | RIS-1 | RIS-2 | RIS-3 | INA-1a | INA-1b | INA-1c | INA-1d | INA-1e | INA-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | Yield * | 1.4 | 3.2 | 2.1 | 2.2 | 2.5 | 4.1 | 3.8 | 4.2 | 0.5 | 1.0 | 1.5 | 0.9 | 1.2 | 0.8 |
| Compound Name | RI-a ** | RI-b ** | ||||||||||||||
| α-Pinene | 934 | 932 | 1.0 | 3.0 | - | 0.4 | 0.5 | 1.8 | 1.2 | 2.0 | - | 1.0 | 2.7 | 0.7 | 1.9 | 1.8 |
| Sabinene | 973 | 969 | - | 0.8 | - | - | - | - | - | - | 1.3 | 2.8 | 3.8 | 2.3 | 3.7 | 6.1 |
| β-Pinene | 979 | 974 | 1.5 | 6.9 | - | - | 0.1 | 2.8 | 1.6 | 1.5 | 1.8 | 2.9 | 6.4 | 3.1 | 4.9 | 5.9 |
| Myrcene | 987 | 988 | - | - | - | - | - | - | - | - | 0.7 | 1.1 | 1.9 | 0.9 | 1.0 | - |
| α-Phellandrene | 1005 | 1002 | - | - | - | - | - | - | - | - | 5.1 | 12.0 | 12.9 | 6.4 | 10.2 | 1.8 |
| p-Cymene | 1025 | 1020 | 1.0 | 0.6 | - | 0.4 | 0.2 | 3.4 | 3.5 | 2.8 | 2.6 | 5.1 | 4.3 | 2.5 | 2.9 | 1.6 |
| Limonene | 1029 | 1024 | - | 1.5 | - | - | - | 0.7 | 1.0 | 1.5 | 2.9 | - | 1.8 | - | - | 3.5 |
| 1,8-Cineole | 1034 | 1031 | - | 24.2 | 24.2 | 34.6 | 33.0 | 21.1 | 18.9 | 16.2 | 36.1 | 44.4 | 43.5 | 41.3 | 49.7 | 50.9 |
| E-Thujone | 1115 | 1112 | - | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| Camphor | 1148 | 1150 | - | - | - | - | - | 0.4 | 0.3 | 0.5 | - | - | - | - | - | - |
| Nonadienal | 1152 | 1150 | - | - | - | - | - | 2.1 | 3.0 | 1.5 | - | - | - | - | - | - |
| Pinocarvone | 1160 | 1160 | - | 0.7 | - | - | - | 0.7 | 0.1 | 0.9 | - | - | - | - | - | - |
| Terpinen-4-ol | 1180 | 1174 | - | 0.7 | - | - | - | - | - | - | 1.5 | 0.8 | 0.9 | 1.1 | 0.7 | - |
| α-Terpineol | 1192 | 1186 | - | - | - | 0.5 | 0.1 | - | - | - | 1.6 | 0.5 | 0.4 | 0.7 | 0.9 | - |
| Myrtenal | 1201 | 1195 | 1.9 | 1.6 | - | - | - | 1.9 | 2.5 | 3.9 | - | - | - | - | - | - |
| Verbenone | 1213 | 1204 | - | 1.9 | - | - | - | 0.4 | 0.1 | 0.8 | - | - | - | - | - | - |
| Hexanoic acid, 1-methyl butyl ester | 1216 | n.f | - | - | - | - | - | 0.6 | 0.8 | 0.7 | - | - | - | - | - | - |
| Terpinyl acetate | 1328 | 1316 | - | - | - | - | - | - | - | - | 1.3 | 1.4 | 0.8 | 2.0 | 0.8 | 1.3 |
| α-Terpineol acetate | 1349 | 1344 | - | - | - | - | - | - | - | - | 19.1 | 9.9 | 9.4 | 16.0 | 8.7 | 12.5 |
| Aromandendrene | 1443 | 1439 | - | - | - | 1.1 | - | - | - | - | - | - | - | - | - | - |
| Alloaromadendrene | 1462 | 1458 | - | - | - | 0.9 | 1.5 | 1.3 | 3.8 | 2.8 | - | - | - | - | - | - |
| Germacrene D | 1486 | 1484 | - | 1.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| β-Selinene | 1489 | 1489 | - | 0.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| γ-Amorphene | 1495 | 1495 | - | 0.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| Bicyclogermacrene | 1504 | 1500 | - | 0.8 | 1.5 | 1.6 | 3.1 | - | - | - | - | - | - | - | - | - |
| Z-Dihydroagarofuran | 1525 | 1519 | - | 15.8 | 39.0 | 23.4 | 23.5 | - | - | - | - | - | - | - | - | - |
| δ-Cadinene | 1527 | 1522 | - | - | - | - | - | - | - | - | 3.4 | 2.5 | 1.6 | 3.1 | 2.0 | - |
| Kessane | 1532 | 1529 | 2.9 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| α-Cadinene | 1535 | 1537 | 4.8 | - | - | - | - | - | - | - | 2.5 | 1.5 | 1.0 | 2.5 | 1.1 | - |
| 10-Epicubebol | 1542 | 1533 | - | - | - | - | - | - | - | - | 0.6 | 0.7 | - | 0.5 | 0.5 | - |
| Maaliol | 1566 | NMR | 70.0 | 34.5 | 33.5 | 32.3 | 36.3 | 38.2 | 40.7 | 40.9 | - | - | - | - | - | - |
| Epiglobulol | 1573 | NMR | - | - | - | - | - | 0.5 | 0.3 | 0.5 | 1.2 | 1.4 | 0.6 | 1.3 | 1.3 | 1.1 |
| Spathulenol | 1578 | 1577 | 2.0 | 1.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Globulol | 1590 | 1590 | 12.8 | 1.0 | 2.0 | 2.8 | 1.6 | 1.1 | 1.1 | 1.1 | - | - | - | - | - | - |
| Viridiflorol | 1601 | 1592 | 2.1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Prostantherol | 1602 | NMR | - | 1.2 | - | 1.9 | - | 21.2 | 19.7 | 16.5 | - | - | - | - | - | - |
| Ledol | 1610 | 1602 | - | - | - | - | - | 1.3 | 0.8 | 4.9 | 1.0 | 0.7 | 0.5 | 0.9 | 0.5 | 0.3 |
| Cubenol, 1,10-di-epi | 1622 | 1618 | - | - | - | - | - | - | - | - | 1.0 | 0.6 | 0.5 | 0.9 | 0.5 | 0.6 |
| Cadinol-epi-alpha | 1648 | 1638 | - | - | - | - | - | - | - | - | 12.0 | 8.2 | 5.4 | 10.8 | 6.8 | 9.4 |
| α-Cadinol | 1661 | 1652 | - | - | - | - | - | - | - | - | 4.3 | 2.5 | 1.7 | 3.1 | 1.8 | 3.2 |
| - | - | Taxon | SUS-1 | STA-1 | MNF-1 | BGB-1 | BGB-2 | GRR-1 | PIA-1 | ASS-1 | TOR-1 | OSF-1 | OSF-2 | OSF-3 | CAA-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | Yield * | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 1.5 | 0.1 | 0.9 | 0.5 | 0.1 | 0.8 |
| Compound Name | RI-a ** | RI-b ** | |||||||||||||
| 2-Hexanol | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.1 | - |
| 2-Hexenal, (E)- | - | - | - | - | - | - | - | - | - | - | - | - | - | 7.9 | - |
| 1-Hexanol | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.1 | - |
| α-Thujene | 928 | 924 | - | - | - | - | - | - | - | - | - | 1.0 | 0.6 | - | - |
| α-Pinene | 934 | 932 | - | - | - | 1.7 | 1.4 | 2.0 | 5.6 | 6.8 | 1.9 | 3.2 | 1.1 | - | 6.3 |
| Camphene | 951 | 946 | - | - | - | - | - | - | - | - | - | 1.2 | - | - | - |
| Verbenene | 956 | 961 | - | - | - | - | - | - | - | - | - | 2.9 | 1.2 | - | - |
| Sabinene | 973 | 969 | - | - | - | - | - | - | - | 0.9 | 0.4 | - | - | - | - |
| β-Pinene | 979 | 974 | 0.5 | - | - | 3.2 | 1.1 | - | 0.9 | 5.9 | 2.8 | 0.4 | 0.7 | - | 12.0 |
| Myrcene | 987 | 988 | - | - | - | - | - | - | - | - | 1.1 | - | - | - | - |
| α-Phellandrene | 1005 | 1002 | - | - | - | - | 1.0 | - | 0.8 | - | 1.2 | 1.4 | 0.5 | - | - |
| p-Cymene | 1025 | 1020 | - | - | - | 1.1 | 1.6 | - | 0.8 | 1.7 | 3.3 | 10.7 | 2.3 | 9.6 | 1.7 |
| Limonene | 1029 | 1024 | - | - | - | 4.4 | 3.2 | 1.2 | 2.3 | 1.6 | 2.8 | 0.5 | - | - | 3.5 |
| 1,8-Cineole | 1034 | 1031 | - | - | - | 82.8 | 82.1 | 66.9 | 42.5 | 42.0 | 38.5 | 30.6 | 51.8 | 5.5 | 72.9 |
| p-Cymenene | 1091 | 1089 | - | - | - | - | - | - | 1.3 | - | - | 0.9 | 0.6 | - | - |
| Linalool | 1101 | 1100 | - | - | 0.7 | - | - | - | 0.5 | - | 23.0 | 0.5 | 0.7 | - | - |
| α-Campholenal | 1129 | 1122 | - | - | - | - | - | - | - | - | - | 0.6 | 0.6 | - | - |
| Z-Verbenol | 1143 | 1137 | - | - | - | - | - | - | - | - | - | 2.8 | 3.0 | 3.4 | - |
| E-Verbenol | 1147 | 1140 | - | - | - | - | - | - | - | - | - | 7.8 | 10.7 | - | - |
| Menth-3-en-8-ol | 1152 | 1145 | - | - | - | - | - | - | - | - | - | 3.6 | 3.2 | - | - |
| Camphor | 1148 | 1150 | - | - | - | - | 1.7 | - | - | - | - | - | - | - | - |
| Pinocarvone | 1160 | 1160 | - | - | 0.6 | - | - | - | - | - | - | 0.3 | - | 2.0 | - |
| Mentha-1,5-dien-8-ol—para | 1170 | 1166 | - | - | - | - | - | - | - | - | - | 10.6 | 9.3 | - | - |
| Santalone | 1179 | 1177 | - | - | - | - | - | - | - | - | - | - | - | - | 1.3 |
| Terpinen-4-ol | 1180 | 1174 | - | 0.8 | - | - | 1.0 | - | 0.6 | 0.4 | 0.9 | 0.6 | 1.2 | 3.9 | - |
| p-Cymen-8-ol | 1187 | 1179 | - | - | - | - | - | - | - | - | - | 1.2 | 1.3 | - | - |
| α-Terpineol | 1192 | 1186 | - | 3.0 | - | - | 1.0 | - | 0.8 | - | 3.6 | 0.3 | - | - | - |
| Myrtenal | 1201 | 1195 | - | - | - | - | - | - | - | - | - | 0.4 | 0.6 | - | - |
| Verbenone | 1213 | 1204 | - | - | - | - | - | - | - | - | - | 1.2 | 1.4 | - | - |
| Linalyl acetate | 1256 | 1254 | - | - | - | - | - | - | - | - | 14.6 | - | 1.6 | - | - |
| Bornyl acetate | 1287 | 1288 | 0.3 | - | 5.7 | - | - | - | - | 0.5 | - | 5.2 | 4.0 | - | - |
| Thymol | 1295 | 1289 | 0.6 | - | 0.3 | - | - | - | - | - | - | 0.3 | 1.0 | 2.8 | - |
| Terpinyl acetate | 1328 | 1316 | 8.2 | 0.6 | - | - | - | - | - | - | - | - | - | - | - |
| α-terpineol acetate | 1349 | 1344 | 0.9 | - | - | - | - | - | - | - | - | 0.8 | 2.4 | - | - |
| Neryl acetate | 1364 | 1359 | 0.3 | - | - | - | - | - | - | - | 0.9 | - | - | - | - |
| Cyclosativene | 1369 | 1369 | 0.9 | 3.6 | - | - | - | - | - | - | - | - | - | - | - |
| α-Copaene | 1373 | 1374 | 11.3 | 5.5 | 0.4 | - | - | - | - | - | - | - | - | - | - |
| Geranyl acetate | 1380 | 1379 | 0.6 | - | 0.2 | - | - | - | - | - | 1.7 | - | - | - | - |
| E-Caryophyllene | 1419 | 1417 | 0.4 | - | 24.8 | - | - | - | 2.5 | - | 1.1 | - | - | - | - |
| β-Copaene | 1434 | 1430 | 0.6 | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| α-Guaiene | 1444 | 1437 | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| Aromandendrene | 1443 | 1439 | - | - | 0.6 | - | - | - | - | - | - | - | - | - | - |
| Alloaromadendrene | 1462 | 1458 | - | - | 1.3 | - | - | - | 0.6 | - | - | - | - | - | 0.7 |
| Dehydroaromadendrane | 1466 | 1460 | - | - | 0.9 | - | - | - | - | - | - | - | - | - | - |
| α-Amorphene | 1481 | 1485 | - | - | 1.7 | - | - | - | 0.5 | - | - | - | - | - | - |
| Germacrene D | 1486 | 1484 | - | - | 2.6 | - | - | - | 1.8 | - | 0.8 | - | - | - | - |
| β-Selinene | 1489 | 1489 | - | - | - | - | - | 3.9 | - | - | - | - | - | - | - |
| γ-Amorphene | 1495 | 1495 | - | - | 0.6 | - | - | - | 1.8 | - | - | - | - | - | - |
| Bicyclogermacrene | 1504 | 1500 | 39.7 | - | 3.9 | 2.8 | 4.3 | 1.7 | 9.6 | - | 0.5 | - | - | - | - |
| α-Farnesene | 1509 | 1505 | - | - | - | - | - | - | - | - | - | - | - | 2.9 | - |
| γ-Cadinene | 1519 | 1513 | - | - | - | - | - | - | - | - | - | - | - | 2.3 | - |
| Z-Dihydroagarofuran | 1525 | 1519 | - | - | - | - | - | 21.4 | - | 15.7 | - | - | - | 3.7 | - |
| δ-Cadinene | 1527 | 1522 | - | - | 1.8 | - | - | - | - | - | - | - | - | 3.5 | - |
| Kessane | 1532 | 1529 | - | 2.3 | 0.6 | - | - | - | - | 0.6 | - | 0.4 | - | 2.5 | - |
| α-Cadinene | 1535 | 1537 | - | 2.2 | 1.9 | - | - | - | - | - | - | - | - | - | - |
| 10-Epicubebol | 1542 | 1533 | 0.2 | - | 13.1 | - | - | - | - | - | - | - | - | 4.0 | - |
| Maaliol | 1566 | NMR | 0.4 | 1.3 | 1.6 | - | - | - | - | 0.6 | - | - | - | 3.8 | - |
| Epiglobulol | 1573 | NMR | 2.0 | 0.6 | - | - | - | - | 3.9 | - | - | - | - | - | - |
| Spathulenol | 1578 | 1577 | 16.4 | 59.3 | 6.0 | 1.0 | 1.6 | - | - | 1.2 | - | - | - | 4.2 | - |
| Caryophyllene oxide | 1584 | 1582 | - | - | 12.1 | 3.0 | - | - | - | - | 0.4 | - | - | - | - |
| Globulol | 1590 | 1590 | 5.6 | 2.3 | - | - | - | 2.9 | 11.4 | 19.8 | 0.5 | 10.5 | - | 9.8 | - |
| Viridiflorol | 1601 | 1592 | - | - | - | - | - | - | - | 1.4 | - | - | - | - | - |
| Prostantherol | 1602 | NMR | 5.6 | - | - | - | - | - | 5.5 | 0.6 | - | - | - | 3.0 | 1.4 |
| Ledol | 1610 | 1602 | 2.2 | - | 5.2 | - | - | - | 4.1 | - | - | - | - | 7.8 | - |
| Cubenol | 1619 | 1618 | 2.6 | 2.8 | - | - | - | - | 0.5 | - | - | - | - | 1.8 | - |
| Alloaromadendrene epoxide | 1638 | 1632 | 0.8 | 4.9 | - | - | - | - | 2.0 | - | - | - | - | - | - |
| Cubenol, 1,10-di-epi | 1622 | 1618 | - | 2.1 | 1.6 | - | - | - | - | - | - | - | - | - | - |
| Cadinol-epi-alpha | 1648 | 1638 | - | 2.5 | 3.1 | - | - | - | - | - | - | - | - | 2.4 | - |
| α-Cadinol | 1661 | 1652 | - | 6.2 | 8.3 | - | - | - | - | - | - | - | - | - | - |
| Cadilene | 1680 | 1675 | - | - | - | - | - | - | - | - | - | - | - | 9.0 | - |
| PEA-1a | PEA-1b | PEA-1c | PEA-1d | PEA-1e | |||
|---|---|---|---|---|---|---|---|
| - | Yield * | 1.7 | 1.9 | 1.2 | 1.1 | 1.6 | |
| RI-a ** | RI-b ** | ||||||
| α-Phellandrene | 1005 | 1002 | - | - | 1.7 | 1.9 | - |
| p-Cymene | 1025 | 1020 | - | 0.7 | 2 | 4 | - |
| Alloaromadendrene | 1464 | 1458 | - | 0.8 | 0.7 | - | - |
| Dehydroaromadendrane | 1466 | 1460 | - | 0.6 | - | - | - |
| α-Amorphene | 1481 | 1485 | 0.8 | 0.7 | 2.1 | 1.7 | 1.1 |
| γ-Amorphene | 1495 | 1495 | 1.2 | 0.6 | 0.9 | 1.1 | - |
| Bicyclogermacrene | 1501 | 1500 | 1.6 | 0.9 | 1.2 | 1.2 | - |
| Kessane | 1534 | 1529 | 18.9 | 22.5 | 18.4 | 17.7 | 18.1 |
| spathulenol | 1574 | 1577 | - | 0.6 | - | 0.7 | - |
| Prostantherol | 1600 | 1600 | 75.6 | 51.7 | 64.6 | 68.5 | 76.9 |
| Ledol | 1604 | 1602 | 1.9 | 17.4 | 1.7 | 1.8 | 1.9 |
| n.d. | 1618 | - | - | - | 4.2 | 1.4 | 1.1 |
| Caryophylla-dien-ol | 1642 | 1644 | - | 1.2 | - | - | 0.8 |
| n.d. | 1664 | - | - | - | 1.3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadgrove, N.J.; Padilla-González, G.F.; Telford, I.R.H.; Greatrex, B.W.; Jones, G.L.; Andrew, R.; Bruhl, J.J.; Langat, M.K.; Melnikovova, I.; Fernandez-Cusimamani, E. Prostanthera (Lamiaceae) as a ‘Cradle of Incense’: Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian ‘Mint Bush’ Species. Plants 2020, 9, 1570. https://doi.org/10.3390/plants9111570
Sadgrove NJ, Padilla-González GF, Telford IRH, Greatrex BW, Jones GL, Andrew R, Bruhl JJ, Langat MK, Melnikovova I, Fernandez-Cusimamani E. Prostanthera (Lamiaceae) as a ‘Cradle of Incense’: Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian ‘Mint Bush’ Species. Plants. 2020; 9(11):1570. https://doi.org/10.3390/plants9111570
Chicago/Turabian StyleSadgrove, Nicholas J., Guillermo F. Padilla-González, Ian R. H. Telford, Ben W. Greatrex, Graham L. Jones, Rose Andrew, Jeremy J. Bruhl, Moses K. Langat, Ingrid Melnikovova, and Eloy Fernandez-Cusimamani. 2020. "Prostanthera (Lamiaceae) as a ‘Cradle of Incense’: Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian ‘Mint Bush’ Species" Plants 9, no. 11: 1570. https://doi.org/10.3390/plants9111570
APA StyleSadgrove, N. J., Padilla-González, G. F., Telford, I. R. H., Greatrex, B. W., Jones, G. L., Andrew, R., Bruhl, J. J., Langat, M. K., Melnikovova, I., & Fernandez-Cusimamani, E. (2020). Prostanthera (Lamiaceae) as a ‘Cradle of Incense’: Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian ‘Mint Bush’ Species. Plants, 9(11), 1570. https://doi.org/10.3390/plants9111570