The Discovery of the Rare Chara baueri (Charales, Charophyceae) in Serbia
Abstract
:1. Introduction
2. Results
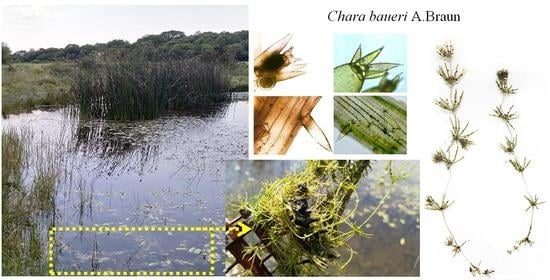
Description of C. Baueri from Serbia
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pukacz, A.; Boszke, P.; Pelechaty, M.J.; Raabe, U. Comparative study of the oospore morphology of two populations of a rare species Chara baueri A. Braun in Cedynia (Poland) and Batzlow (Germany). Acta Soc. Bot. Pol. 2012, 81, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Raabe, U. Chara baueri rediscovered in Germany—Plus additional notes on Gustav Heinrich Bauer (1794–1888) and his herbarium. Int. Res. Group Charophytes News 2009, 20, 13–15. [Google Scholar]
- Krause, W. Charales (Charophyceae) Süßwasserflora von Mitteleuropa; Gustav Fischer Verlag: Jena, Germany, 1997. [Google Scholar]
- Langangen, A.; Sviridenko, B.F. Chara baueri A. Br., a charophyte with a disjunct distribution. Cryptogam. Algol. 1995, 16, 125–132. [Google Scholar]
- Pukacz, A.; Pelechaty, M.; Raabe, U. Pierwsze stanowisko Chara baueri (Characeae) w Polsce. Fragm Florist Geobot Pol. 2009, 16, 425–429. [Google Scholar]
- Urbaniak, J.; Gąbka, M. Polish Charophytes. An Illustrated Guide to Identification; Universytet Przyrodiczy We Wroclawiu: Wroclaw, Poland, 2014; ISBN 978-83-7717-166-0. [Google Scholar]
- Klinkova, G.Y.; Zhakova, L.V. New and rare species Charales in the flora of the lower Volga region. Byull. Moskovsk. Obshch. Isp. Prir. Otd. Biol. 2014, 119, 61–66. [Google Scholar]
- Noedoost, F.; Riahi, H.; Sheidai, M.; Ahmadi, A. Distribution of Charophytes from Iran with three new records of Characeae (Charales, Chlorophyta). Cryptogam. Algol. 2015, 36, 389–405. [Google Scholar] [CrossRef]
- Schneider, S.C.; Nowak, P.; von Ammon, U.; Ballot, A. Species differentiation in the genus Chara (Charophyceae): Considerable phenotypic plasticity occurs within homogenous genetic groups. Eur. J. Phycol. 2016, 51, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Reichenbach, H.G.L. Dr. Joh. Christ. Mössler’s Handbuch der Gewächskunde, Enthaltend eine Flora von Deutschland mit Hinzufügung der Wichtigsten Ausländischen Cultur-Pflanzen, 2nd ed.; Johann Friedrich Hammerich: Altona, Germany, 1829; Volume 3, pp. 1592–1601. [Google Scholar]
- Braun, A. Uebersicht der genauer bekannten Chara-Arten. Flora Allg. Bot. Ztg. 1835, 18, 49–73. [Google Scholar]
- Braun, A.; Nordstedt, C.F.O. Fragmente einer Monographie der Characeen; Königlichen Akademie der Wissenschaften: Berlin, Germany, 1882; pp. 1–211. [Google Scholar]
- Ganterer, U. Die Bisher Bekannten Österreichischen Charen: Vom Morphologischen Standpunkte Bearbeitet; Carl Haas’schen Verlag: Vienna, Austria, 1847. [Google Scholar]
- Migula, W. Die Characeen Deutschlands, Oesterreichs und der Schweiz: Unter Berücksichtigung aller Arten Europas; E. Kummer: Leipzig, Germany, 1897. [Google Scholar]
- Hutorowicz, A. Morphological variability of oospores of Chara baueri A. Braun [Characeae]. Acta Soc. Bot. Pol. 2007, 76, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.D.; Imahori, K. A Revision of the Characeae. First Part:Monograph of the Characeae; J. Cramer Verlag: Weinheim, Germany, 1965. [Google Scholar]
- Гoллербах, М.М.; Красавина, Л.К. Харoвые Вoдoрoсли. Определитель Преснoвoдных Вoдoрoслей СССР; Nauka: Leningrad, Russia, 1983. [Google Scholar]
- Google Earth. Available online: https://earth.google.com/web/search/Sankt+Andr%c3%a4,+Austria/@45.81280243,18.96052335,83.72340015a,114.48633148d,35y,0h,0t,0r/data=CigiJgokCQAz16vOpUdAEdpVE1pBRUdAGQnvaQoHsjBAIfzZ_8Vy3CxA (accessed on 26 October 2020).
- Becker, R. Gefährdung und Schutz von Characeen. In Armleuchteralgen. Die Characeen Deutschlands; Deutschlands, A.C., Ed.; Springer: Heidelberg, Germany, 2016; pp. 149–191. ISBN 978-3-662-47796-0. [Google Scholar]
- Doege, A.; van de Weyer, K.; Becker, R.; Schubert, H. Bioindikation mit Characeen. In Armleuchteralgen. Die Characeen Deutschlands; Deutschlands, A.C., Ed.; Springer: Heidelberg, Germany, 2016; pp. 149–191. ISBN 978-3-662-47796-0. [Google Scholar]
- Gregor, T. Chara baueri. In Armleuchteralgen. Die Characeen Deutschlands; Deutschlands, A.C., Ed.; Springer: Heidelberg, Germany, 2016; pp. 247–248. ISBN 978-3-662-47796-0. [Google Scholar]
- Trbojević, I.; Marković, A.; Blaženčić, J.; Subakov Simić, G.; Nowak, P.; Ballot, A.; Schneider, S. Genetic and morphological variation in Chara contraria and a taxon morphologically resembling Chara connivens. Bot. Lett. 2020, 167, 187–200. [Google Scholar] [CrossRef]
- Simić, D.; Puzović, S. Ptice Srbije i Područja od Međunarodnog Značaja; Liga za ornitološku akciju Srbije: Belgrade, Serbia, 2008; ISBN 978-86-911303-0-5. [Google Scholar]
- BirdLife International (2020) Species factsheet: Anser Anser. Available online: http://datazone.birdlife.org/species/factsheet/greylag-goose-anser-anser/distribution (accessed on 4 September 2020).
- Dick, G.; Baccetti, N.; Boukhalfa, D.; Darolova, A.; Faragó, S.; Hudec, K.; Leito, A.; Markkola, J.; Witkowski, J. Graylag Goose Anser anser: Central Europe/North Africa. In Goose Populations of the Western Palearctic. A Review of Status and Distribution; Madsen, J., Cracknell, G., Fux, T., Eds.; Wetlands International Publication: Wageningen, The Netherlands; National Environmental Research Institute: Rønde, Denmark, 1999; pp. 202–213. ISBN 87-7772-437-2. [Google Scholar]
- Wood, R.D.; Imahori, K. A Revision of the Characeae. Second Part: Iconograph of the Characeae; J. Cramer Verlag: Weinheim, Germany, 1964. [Google Scholar]




| T | O2 | O2 | pH | Cond | °dH | Ca2+ | Mg2+ | NO2- | NO3- | NH4+ | TN | OP | TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | mg/L | % | µS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |||
| POND 1 | ||||||||||||||
| May 2018 | 20.5 | 9.05 | 102 | 8.23 | 325 | - | - | - | - | - | - | - | - | - |
| June 2018 | 22 | 4.42 | 50.5 | 8 | 300 | - | - | - | - | - | - | - | - | - |
| July 2018 | 30.7 | 15.45 | 190 | 9.35 | 338 | 1.1 | 3.7 | 2.7 | 0.021 | <0.5 | 0.24 | 2.5 | <0.020 | 0.028 |
| August 2018 | 31 | 12 | 162 | 8.76 | 379 | 2.6 | 8.8 | 5.7 | <0.020 | <0.5 | 0.23 | 1.9 | <0.020 | 0.026 |
| September 2018 | 14.5 | 9.94 | 101.2 | 8.68 | 346 | 6.3 | 30.2 | 9.1 | 0.027 | <0.5 | <0.05 | 2.9 | <0.020 | 0.031 |
| May 2019 | 18 | 15 | 152.6 | 9.61 | 328 | 5.2 | 12 | 15 | <0.005 | <0.5 | 2.2 | 1.2 | <0.010 | 0.24 |
| June 2019 | 29.15 | 11.9 | 157.5 | 9.13 | 344 | 3.7 | 11.6 | 9 | <0.005 | <0.5 | 0.45 | 1.5 | <0.010 | 0.07 |
| July 2019 | 28.3 | 2.57 | 32.4 | 8.13 | 445 | 6.1 | 23.2 | 12.4 | <0.005 | <0.5 | 0.54 | 1.9 | <0.010 | 0.07 |
| POND 2 | ||||||||||||||
| May 2018 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| June 2018 | 21 | 2.21 | 25.4 | 7.56 | 275 | - | - | - | - | - | - | - | - | - |
| July 2018 | 28.1 | 7.5 | 96.5 | 8.12 | 342 | 6.1 | 31.2 | 7.5 | 0.043 | <0.5 | 0.21 | 1.7 | <0.020 | 0.029 |
| August 2018 | 26.1 | 6.24 | 77.1 | 8.44 | 435 | 4.4 | 21.8 | 5.9 | <0.020 | <0.5 | 0.18 | 3 | <0.020 | 0.046 |
| September 2018 | 15 | 12 | 120 | 9.08 | 355 | 6.7 | 33.8 | 8.4 | 0.08 | 0.91 | 0.79 | 5.3 | <0.020 | 0.04 |
| May 2019 | 15.5 | 13.7 | 135.2 | 9.18 | 352 | - | - | - | - | - | - | - | - | - |
| June 2019 | 28 | 3.63 | 43.6 | 7.96 | 354 | - | - | - | - | - | - | - | - | - |
| July 2019 | 25.6 | 2.42 | 28.4 | 8.13 | 416 | - | - | - | - | - | - | - | - | - |
| Literature Source | Reichenbach, 1829 [10] | Braun, 1835 [11] Braun and Nordstedt, 1882 [12] | Ganterer, 1847 [13] | Migula, 1897 [14] | Langangen and Sviridenko, 1995 [4] | Hutorowicz, 2007 [15] | Pukacz, 2012 [1] | Urbaniak and Gąbka, 2014 [6] | Specimens from Serbia |
|---|---|---|---|---|---|---|---|---|---|
| Note | Specimen from Kazakhstan (1994)/ Specimen from Sweden (1849), herbarium specimen examined in dry condition | Oospores from specimens from Kazakhstan [4] | Oospores from specimens from Cedynia, Western Poland (August 2008)/ Oospores from specimens from near Batzlow, Germany (2006) | ||||||
| Habitus description | Nice green color, turning black towards the bottom. Stiff stature, almost cartilaginous, ‘’shining’’ similarly to Ceratophyllum. | Hard to differentiate from Ch. coronata (Chara braunii) without the loupe | Richly branched, bushy habitus, mostly light green in color | Habitus is not easy to distinguish from Ch. coronata (Chara braunii), all parts are proportionally thicker. Light yellow-green or green, rarely brownish-green. | -/- | - | - | Rather small plants, richly branched, yellow green to light green, strongly resembling C.braunii | Light green to yellowish green. Resembling C. braunii, but branchlets more rounded, plump and voluminous—like succulent. |
| Axes | |||||||||
| 1. Incrustation | - | Not incrusted | - | Incrustation is abundant | Not incrusted/ slightly incrusted | - | - | Slightly incrusted | Very slightly incrusted |
| 2. Diameter | More than 1.0975 mm (more than ½ Linie, 1 Linie = 2.195 mm) | Up to 1 mm | Up to 0.57 (0.65 in Figure 2 caption) mm/0.93 mm | 0.6–2.1 mm | 0.55–0.95 mm | ||||
| Cortex | - | Finely striped cortex | In the uppermost internodes, or throughout the stem more or less clearly and finely striped, and covered with scattered spines | Triplostichous, isostichous | 2–3 corticate, isostichous, young internodes mostly 2 corticate/ Mostly 2 corticate, isostichous (hard to determine in dry material) | - | - | Triplostichous, in some parts irregular | Triplostichous, isostichous cortex developed throughout the stem, in some parts irregular |
| Spine cells | |||||||||
| 1. Description | - | Fine, spiked | - | Solitary, up to ½ of axes diameter, acuminate–spiky, especially in young segments | Solitary, acute, to 1 x stem diameter, commonly shorter, many on young internodes/ Solitary, acute, many in young internodes, only few in the lower parts of stem | - | - | Solitary, common, acuminate, not exceeding axes diameter | Large, solitary, acuminate, more numerous in the upper section of axes |
| Branchlets | |||||||||
| 1. Number in a whorl and description | - | Ecorticated | Ecorticated | 8–9 | 8–9, ecorticated/ 7–8, ecorticated | - | - | 7 | 8–11, ecorticated |
| Oogonia | |||||||||
| 1. Number in node | Usually geminate, rarely 1 or 3 | - | Single, geminate or 3, ovoid | - | - | - | - | - | 1, mostly 2, sometimes even 3 |
| 2. Dimensions (length × width) | - | 700–880 µm × 380–480 µm | - | 650 × 500 µm | 1000 µm/ 600 µm × 250 µm (immature) | - | - | 585–950 µm × 365–610 µm | 580–710 (775) µm × 370–450 µm |
| 3. Number of convolutions | 8–10 | 10–11 | Mostly 10 | 8–10 | - | - | - | - | 9–10 |
| 4. Coronula width × height | - | 140–200 µm × 220–250 µm | 150–200 µm | - | - | - | - | (120) 170–260 (275) µm × (150) 200–330 (350) µm | |
| Oospore | |||||||||
| 1. Color | - | - | - | Black | -/Black | Light brown to black/- | Dark brown or black/- | Black/dark brown | Black |
| 2. Dimensions (length × width) | - | 500–560 µm × 310–350 µm | - | 500–550 µm × 280–340 µm | -/600 µm | 436–574 µm × 281–340 µm | 400–667 µm × 183–300 µm/417–550 µm × 216–300 µm | 465–740 µm × 280–340 µm | 475–620 µm × 230–350 µm |
| 3. Ridges description | - | - | - | Irregular, blunt/sharp edges | - | Unpronounced in young and prominent in mature oospores | Prominent ridges/- | - | Oval, blunt |
| 4. Ridges number | - | - | - | 8 | - | 8–11 | 8–11 (most often 9)/8–11 | - | 8–10 (11) |
| 5. Fossa | - | - | - | - | - | - | - | - | 50–70 µm |
| 6. Membrane coloration | - | - | - | - | - | - | - | - | Light brown |
| 7. Membrane structure | - | - | - | - | - | Either smooth or finely granulated | - | - | Finely granulated |
| Antheridia | |||||||||
| Diameter and description | In pairs or individual, brick red color | 280–370 µm | Individual or in pairs, beneath the oogonia | 250–300 µm | -/200 µm | - | - | 250–330 µm, below oogonia | 250–300 µm, below oogonia, individual, brick red color |
| O2 (mg/L) | Hardness (°dH) | pH | Conductivity (µS/cm) | Mg2+ (mg/L) | Ca2+ (mg/L) | TP (mg/L) | PO4− (mg/L) | TN (mg/L) | NO2− (mg/L) | NO3− (mg/L) | NH4+ (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pukacz et al., 2012 [1] | ||||||||||||
| Cediniya (Poland) | 6.14 | 12.9 | 8.01 | 611 | 14.9 | 71.7 | 1.04 | 0.63 | 3.89 | - | 0.47 | 1.31 |
| Batzlow (Germany) | 3.25 | 13.2 | 7.92 | 632 | 16.3 | 67.6 | 1.12 | 0.71 | 5.15 | 0.02 | 0.68 | 1.45 |
| This study | ||||||||||||
| Pond 1 | 10.04 | 4.17 | 8.7 | 350.6 | 8.98 | 14.92 | 0.08 | <0.015 | 1.98 | 0.02 | <0.5 | 0.73 |
| Pond 2 | 6.8 | 5.73 | 8.3 | 361.3 | 7.27 | 28.93 | 0.04 | <0.02 | 3.33 | 0.06 | 0.91 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trbojević, I.; Milovanović, V.; Subakov Simić, G. The Discovery of the Rare Chara baueri (Charales, Charophyceae) in Serbia. Plants 2020, 9, 1606. https://doi.org/10.3390/plants9111606
Trbojević I, Milovanović V, Subakov Simić G. The Discovery of the Rare Chara baueri (Charales, Charophyceae) in Serbia. Plants. 2020; 9(11):1606. https://doi.org/10.3390/plants9111606
Chicago/Turabian StyleTrbojević, Ivana, Vanja Milovanović, and Gordana Subakov Simić. 2020. "The Discovery of the Rare Chara baueri (Charales, Charophyceae) in Serbia" Plants 9, no. 11: 1606. https://doi.org/10.3390/plants9111606
APA StyleTrbojević, I., Milovanović, V., & Subakov Simić, G. (2020). The Discovery of the Rare Chara baueri (Charales, Charophyceae) in Serbia. Plants, 9(11), 1606. https://doi.org/10.3390/plants9111606