Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea
Abstract
:1. Introduction
2. Results
2.1. Plant Biomass Production
2.2. Nitrate, Ammonium, Amino Acids, and Soluble Protein
2.3. Activity and Expression of Marker Enzymes for N Assimilation
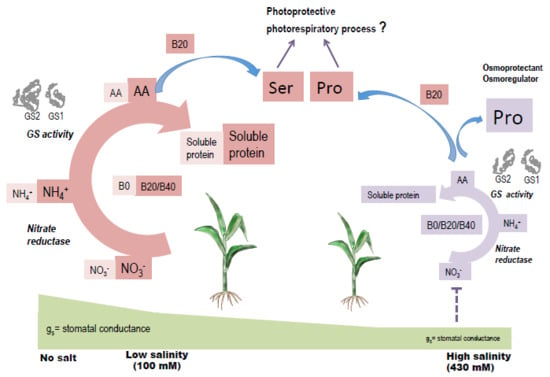
3. Discussion
3.1. Boron Improves N Assimilation
3.2. Under Low Salinity N Assimilation Exceeds Plant Demand
3.3. Moderate B Levels Favour Amino Acid Synthesis and Soluble Protein Content Under Saline Conditions
4. Materials and Methods
4.1. Growth Conditions and Experimental Design
4.2. Stomatal Conductance, Growth Parameters
4.3. Determination of Metabolites: Nitrate, Ammonium, and Amino Acids
4.4. Enzymatic Assays
4.5. Gel Electrophoresis, the Gel-Staining Procedure, and Protein Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bastías, E.; González-Moro, M.B.; González-Murua, C. Zea mays L. amylacea from the Lluta Valley (Arica-Chile) tolerates salinity stress when high levels of boron are available. Plant Soil 2004, 267, 73–84. [Google Scholar] [CrossRef]
- Carvajal, M.; Martínez, V.; Alcaraz, C.F. Physiological function of water channels as affected by salinity in roots of paprika pepper. Physiol. Plant. 1999, 105, 95–101. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Bastías, E.; Zhu, C.; Schäffner, A.R.; González-Moro, M.B.; González-Murua, C.; Carvajal, M. Boric acid and salinity effects on maize roots. Response of aquaporins ZmPIP1 and ZmPIP2, and plasma membrane H+-ATPase, in relation to water and nutrient uptake. Physiol. Plant. 2008, 132, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Gouia, H.; Ghorbal, M.H.; Touraine, B. Effects of NaCl on flows of N and mineral ions and on NO3− reduction rate within whole plants of salt-sensitive bean and salt-tolerant cotton. Plant Physiol. 1994, 105, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Debouba, M.; Gouia, H.; Suzuki, A.; Ghorbel, M.H. NaCl stress effects on enzyme involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedlings. J. Plant Physiol. 2006, 163, 1247–1258. [Google Scholar] [CrossRef]
- Wege, S.; Jossier, M.; Filleur, S.; Thomine, S.; Barbier-Brygoo, H.; Gambale, F.; De Angeli, A. The proline 160 in the selectivity filter of the Arabidopsis NO3−/H+ exchanger AtCLCa is essential for nitrate accumulation in planta. Plant J. 2010, 63, 861–869. [Google Scholar] [CrossRef]
- Zifarelli, G.; Pusch, M. CLC transport proteins in plants. FEBS Lett. 2010, 584, 2122–2127. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhao, F.G.; Tang, R.J.; Yu, Y.; Song, J.; Wang, Y.; Li, L.; Luan, S. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2036–E2045. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.M.F.; Salama, K.H.A. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122. [Google Scholar] [CrossRef]
- Botrel, A.; Kaiser, W.M. Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status. Planta 1997, 201, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Abd-El Baki, G.K.; Siefritz, F.; Man, H.M.; Weiner, H.; Kaldenhoff, R.; Kaiser, W.M. Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ. 2000, 23, 515–521. [Google Scholar] [CrossRef]
- Bourgeais-Chaillou, P.; Pérez-Alfocea, F.; Guerrier, G. Comparative effects of N-sources on growth and physiological responses of soyabean exposed to NaCl-stress. J. Exp. Bot. 1992, 43, 1225–1233. [Google Scholar] [CrossRef]
- Sahu, A.C.; Sahoo, S.K.; Sahoo, N. NaCl-stress induced alteration in glutamine synthetase activity in excised senescing leaves of a salt-sensitive and a salt-tolerant rice cultivar in light and darkness. Plant Growth Regul. 2001, 34, 287–292. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, Y.T.; Kao, C.H. The effect of NaCl on proline accumulation in rice leaves. Plant Growth Regul. 2002, 36, 275–285. [Google Scholar] [CrossRef]
- Debouba, M.; Maaroufi-Dghimi, H.; Suzuki, A.; Ghorbel, M.H.; Gouia, H. Changes in growth and activity of enzymes involved in nitrate reduction and ammonium assimilation in tomato seedlings in response to NaCl stress. Ann. Bot. 2007, 99, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Q.; Yuan, Y.Z.; Ou, J.Q.; Lin, Q.H.; Zhang, C.F. Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. J. Plant Physiol. 2007, 164, 695–701. [Google Scholar] [CrossRef]
- Zhonghua, T.; Yanju, L.; Xiaorui, G.; Yuangang, Z. The combined effects of salinity and nitrogen forms on Catharanthus roseus: The role of internal ammonium and free amino acids during salt stress. J. Plant Nutr. Soil Sci. 2011, 174, 135–144. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Melo, A.R.B.; Viégas, R.A.; Oliveira, J.T.A. Salinity-induced effects on nitrogen assimilation related to growth in cowpea plants. Environ. Exp. Bot. 2001, 46, 171–179. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Shim, I.S.; Kobayashi, K.; Usui, K. Regulation of ammonium accumulation during salt stress in rice (Oryza sativa L) seedlings. Plant Prod. Sci. 2005, 8, 397–404. [Google Scholar] [CrossRef]
- Veeranagamallaiah, G.; Chandraobulreddy, P.; Jyothsnakumari, G.; Sudhakar, C. Glutamine synthetase expression and pyrroline-5-carboxylate reductase activity influence proline accumulation in two cultivars of foxtail millet (Setaria italica L) with different salt sensitivity. Environ. Exp. Bot. 2007, 60, 239–244. [Google Scholar] [CrossRef]
- Kant, S.; Kant, P.; Lips, H.; Barak, S. Partial substitution of NO3− by NH4+ fertilization increases ammonium assimilating enzymes activities and reduces the deleterious effects of salinity on the growth of barley. J. Plant Physiol. 2007, 164, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, M.B.; González-Fontes, A.; Rexach, J.; Camacho-Cristóbal, J.J.; Maldonado, J.M.; Navarro-Gochicoa, M.T. Role of boron in vascular plants and response mechanisms to boron stresses. Plant Stress 2010, 4, 115–122. [Google Scholar]
- Ruiz, J.M.; Lopez-Lefebre, L.R.; Sanchez, E.; Rivero, R.M.; García, P.C.; Romero, L. Preeliminary studies on the influence of boron on the foliar biomass and quality of tobacco leaves subjected to NO3− fertilisation. J. Sci. Food Agric. 2001, 81, 739–744. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 2007, 226, 443–451. [Google Scholar] [CrossRef]
- Redondo-Nieto, M.; Rivilla, R.; El-Hamdaoui, A.; Bonilla, I.; Bolaños, L. Boron deficiency affects early infection events in the pea-Rhizobium symbiotic interaction. Aust. J. Plant Physiol. 2001, 28, 819–823. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in plants: Deficiency and toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Navarro-Gochicoa, M.T.; Rexach, J.; González-Fontes, A.; Herrera-Rodríguez, M.B. Plant response to boron deficiency and boron use efficiency in crop plants. In Plant Micronutrients Use Efficiency; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Levene, T.R.; Hullett, C.R. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum. Commun. Res. 2002, 28, 612–625. [Google Scholar] [CrossRef]
- Hayes, J.E.; Reid, J.R. Boron tolerance in barley is mediated by efflux of boron from the roots. Plant Physiol. 2004, 136, 3376–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, K.; Fujiwara, T. Boron transport in plants: Co-ordinated regulation of transporters. Ann. Bot. 2010, 105, 1103–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Lefebre, L.R.; Ruiz, J.M.; Rivero, R.M.; García, P.C.; Sánchez, E.; Romero, L. Supplemental boron stimulates ammonium assimilation in leaves of tobacco plants (Nicotiana tabacum L.). Plant Growth Reg. 2002, 26, 231–236. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency causes a drastic decrease in nitrate content and nitrate reductase activity, and increases the content of carbohydrates in leaves from tobacco plants. Planta 1999, 209, 528–536. [Google Scholar] [CrossRef]
- Cervilla, L.M.; Blasco, B.; Ríos, J.J.; Rosales, M.A.; Rubio-Wilhelmi, M.M.; Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J.M. Response of nitrogen metabolism to boron toxicity in tomato plants. Plant Biol. 2009, 11, 671–677. [Google Scholar] [CrossRef]
- Shen, Z.; Liang, Y.C.; Shen, K. Effect of boron on the nitrate reductase activity in oilseed rape plants. J. Plant Nutr. 1993, 16, 1229–1239. [Google Scholar] [CrossRef]
- Ramón, A.M.; Carpena-Ruiz, R.O.; Gárate, A. In vitro stabilization and distribution of nitrate reductase in tomato plants. Incidence of boron deficiency. J. Plant Physiol. 1989, 135, 126–128. [Google Scholar]
- Teixeira, J.; Fidalgo, F. Salt stress affects glutamine synthetase activity and mRNA accumulation on potato plants in an organ-dependent manner. Plant Physiol. Biochem. 2009, 47, 807–813. [Google Scholar] [CrossRef]
- Leidi, E.O.; Lips, S.H. The effect of NaCl salinity on photosynthesis, 14C-translocation and yield in wheat plants irrigated with ammonium or nitrate solutions. Irrig. Sci. 1990, 11, 155–161. [Google Scholar] [CrossRef]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Fuggi, A. Nitrate reductase in durum wheat seedlings as affected by nitrate nutrition and salinity. Funct. Plant Biol. 2005, 32, 209–219. [Google Scholar] [CrossRef]
- Hirel, B.; Martín, A.; Tercé-Laforgue, T.; González-Moro, M.B.; Estavillo, J.M. Physiology of maize I: A comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiol. Plant 2005, 124, 167–177. [Google Scholar] [CrossRef]
- Gautam, S.; Singh, P. Salicylic acid-induced salinity tolerance in corn grown under NaCl stress. Acta Physiol. Plant. 2009, 31, 1185–1190. [Google Scholar] [CrossRef]
- Kamachi, K.; Yamaya, T.; Hayakawa, T.; Mae, T.; Ojima, K. Changes in cytosolic glutamine synthetase polypeptide and its RNA in leaf blade of rice plants during natural senescence. Plant Physiol. 1992, 98, 1323–1329. [Google Scholar] [CrossRef]
- Brugière, N.; Dubois, F.; Limami, A.; Lelandais, M.; Roux, Y.; Sangwan, R.S.; Hirel, B. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell 1999, 11, 1995–2011. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Belastegui-Macadam, X.; Quilleré, I.; Floriot, M.; Valadier, M.H.; Pommel, B.; Andrieu, B.; Donnison, I.; Hirel, B. Nitrogen management and senescence in two maize hybrids differing in the persistence of leaf greenness: Agronomic, physiological and molecular aspects. New Physiol. 2005, 167, 483–492. [Google Scholar] [CrossRef]
- Teixeira, J.; Pereira, S.; Queirós, F.; Fidalgo, F. Specific roles of potato glutamine synthetase isoenzymes in callus tissue grown under salinity: Molecular and biochemical responses. Plant Cell Tiss. Organ Cult. 2006, 87, 1–7. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Viégas, R.A.; Rocha, I.M.A.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Oliveira, J.O.A. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J. Plant Physiol. 2003, 160, 115–123. [Google Scholar] [CrossRef]
- Pang, Q.; Chen, S.; Dai, S.; Chen, Y.; Wang, Y.; Yan, X. Comparative proteomics of salt tolerance in Arabidopsis thaliana and Thellungiella halophila. J. Proteome Res. 2010, 9, 2584–2599. [Google Scholar] [CrossRef]
- Kumar-Swami, A.; Alam, S.I.; Sengupta, N.; Sarin, R. Differential proteomic analysis of salt response in Sorghum bicolor leaves. Environ. Exp. Bot. 2011, 71, 321–328. [Google Scholar] [CrossRef]
- Di Martino, C.; Delfine, S.; Pizzuto, R.; Loreto, F.; Fuggi, A. Free amino acids and glycine betaine in leaf osmoregulation of spinach responding salt stress. New Phytol. 2003, 158, 455–463. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Hoshida, H.; Tanaka, Y.; Hibino, T.; Hayashi, Y.; Tanaka, A.; Takabe, T.; Takabe, T. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol. Biol. 2000, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- González-Moro, M.B.; Loureiro-Beldarrain, I.; Estavillo, J.M.; Duñabeitia, M.K.; Muñoz-Rueda, A.; González-Murua, C. Effect of photorespiratory C2 acids on CO2 assimilation, PS II photochemistry and the xanthophyll cycle in maize. Photosynth. Res. 2003, 78, 161–173. [Google Scholar] [CrossRef]
- Ranieri, A.; Bernardi, R.M.; Lanese, P.; Soldatini, G.F. Changes in free amino acid content and protein pattern of maize seedlings under water stress. Environ. Exp. Bot. 1989, 29, 251–457. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue metabolic responses to salt stress in wild and cultivated barley. PLoS ONE 2013, 8, e55431. [Google Scholar] [CrossRef]
- Berteli, F.; Corrales, E.; Guerrero, C.; Ariza, M.J.; Pliego, F.; Valpuesta, V. Salt stress increases ferredoxin-dependent glutamate synthase activity and protein level in the leaves of tomato. Physiol. Plant. 1995, 93, 259–264. [Google Scholar] [CrossRef]
- Lutts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- Ferguson, L.; Poss, J.A.; Grattan, S.R.; Grieve, C.M.; Wang, D.; Wilson, C.; Donovan, T.J.; Chao, C.T. Pistachio rootstocks influence scion growth and ion relations under salinity and boron stress. J. Am. Soc. Hortic. Sci. 2002, 127, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Grattan, S.R.; Shannon, M.C.; Grieve, C.M.; Poss, J.A.; Suarez, D.L.; Leland, F. Interactive effects of salinity and boron on the performance and water use of eucalyptus. Acta Hortic. 1996, 449, 607–613. [Google Scholar] [CrossRef]
- Holloway, R.E.; Alston, A.M. The effects of salt and boron on growth of wheat. Aust. J. Agric. Res. 1992, 43, 987–1001. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A. Wheat response to interactive effects of boron and salinity. J. Plant Nutr. 2000, 23, 1217–1226. [Google Scholar] [CrossRef]
- Alpaslan, M.; Gunes, A. Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 2001, 236, 123–128. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Bassil, E.S.; Brown, P.H.; Läuchli, A. Boron response in wheat is genotype-dependent and related to boron uptake, translocation, allocation, plant phenological development and growth rate. Funct. Plant Biol. 2005, 32, 507–515. [Google Scholar] [CrossRef]
- Huanca-Mamani, W.; Arias-Carrasco, R.; Cárdenas-Ninasivincha, S.; Rojas-Herrera, M.; Sepúlveda-Hermosilla, M.; Caris-Maldonado, J.C.; Bastías, E.; Maracaja-Coutinho, V. Long non-coding RNAs responsive to salt and boron stress in the hyper-arid Lluteño maize from Atacama Desert. Genes 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Moro, M.B.; Lacuesta, M.; Royuela, M.; Muñoz-Rueda, A.; González-Murua, C. Comparative study of the inhibition of photosynthesis caused by aminooxyacetic acid and phosphinothricin in Zea mays. J. Plant Physiol. 1993, 142, 161–166. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitrification of salicylic acid. Comm. Soil Sci. Plant Anal. 1975, 6, 71–90. [Google Scholar] [CrossRef]
- O’Neal, D.; Joy, K.W. Glutamine synthetase of pea leaves. I. Purification, stabilization and pH optima. Arch. Biochem. Biophys. 1973, 59, 113–122. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]






| Leaves DW | Stem DW | Root DW | Ammonium | Nitrate | Protein | Total AA | Asp+Asn | Ser | ||||||||||
| sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | |
| Boron | ns | 0.084 | * | 0.38 | ns | 0.13 | * | 0.237 | ** | 0.504 | *** | 0.419 | *** | 0.521 | *** | 0.374 | *** | 0.563 |
| Salinity | *** | 0.801 | *** | 0.858 | *** | 0.644 | ns | 0.018 | *** | 0.835 | *** | 0.258 | *** | 0.79 | ** | 0.733 | *** | 0.752 |
| Boron * Salinity | ns | 0.258 | * | 0.495 | ns | 0.191 | ns | 0.227 | *** | 0.658 | *** | 0.56 | *** | 0.629 | *** | 0.393 | *** | 0.737 |
| Gly | Ala | Pro | Glu+Gln | NR real | NR max | GS activity | GS2 | GS1 | ||||||||||
| sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | sig | partial η2 | |
| Boron | *** | 0.651 | ** | 0.34 | *** | 0.535 | ns | 0.084 | ns | 0.032 | ns | 0.017 | ns | 0.032 | *** | 0.977 | *** | 0.99 |
| Salinity | *** | 0.664 | *** | 0.892 | *** | 0.772 | *** | 0.679 | *** | 0.485 | * | 0.173 | ** | 0.288 | *** | 0.988 | *** | 0.988 |
| Boron * Salinity | *** | 0.803 | *** | 0.665 | *** | 0.637 | ns | 0.315 | ns | 0.099 | ns | 0.044 | ns | 0.166 | *** | 0.974 | *** | 0.995 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M.B. Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants 2020, 9, 322. https://doi.org/10.3390/plants9030322
Fuertes-Mendizábal T, Bastías EI, González-Murua C, González-Moro MB. Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants. 2020; 9(3):322. https://doi.org/10.3390/plants9030322
Chicago/Turabian StyleFuertes-Mendizábal, Teresa, Elizabeth Irica Bastías, Carmen González-Murua, and Mª Begoña González-Moro. 2020. "Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea" Plants 9, no. 3: 322. https://doi.org/10.3390/plants9030322
APA StyleFuertes-Mendizábal, T., Bastías, E. I., González-Murua, C., & González-Moro, M. B. (2020). Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants, 9(3), 322. https://doi.org/10.3390/plants9030322