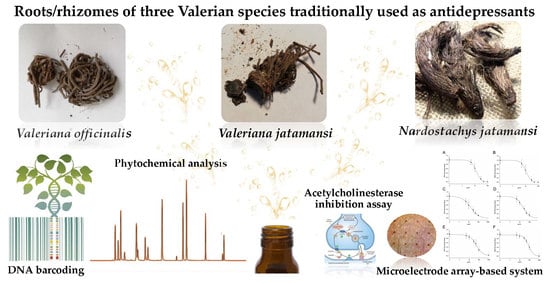
Comparative and Functional Screening of Three Species Traditionally used as Antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D.Don) DC.
Abstract
:1. Introduction
2. Results
2.1. DNA Barcoding Studies
2.2. Macro- and Micro-Morphological Studies
2.3. Phytochemical Characterization
2.4. Acetylcholinesterase Inhibitory Activity
2.5. Neuroactive Effects
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Essential Oils Preparation
4.3. Species Identification by DNA Barcoding
4.4. Micromorphological Characterization
4.5. Phytochemical Analyses
4.6. Acetylcholinesterase Inhibition Assay
4.7. Microelectrode Array Analysis
4.7.1. Primary Mouse Cortical Neuronal Cultures
4.7.2. Data Recordings, Signal Processing and Data Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.K.; Satyal, P.; Setzer, W.N. Himalayan Aromatic Medicinal Plants: A Review of their Ethnopharmacology, Volatile Phytochemistry, and Biological Activities. Medicines 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrashedy, N.A.; Molina, J. The ethnobotany of psychoactive plant use: A phylogenetic perspective. PeerJ 2016, 4, e2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, S.K.; Thakur, R. Herbal Resources of India and Nepal. Tonic, Memory Boosters and Mood Swing; Scientific Publishers: Jodhpur, India, 2016. [Google Scholar]
- Polya, G. Biochemical Targets of Plant Bioactive Compounds: A Pharmacological Reference Guide to Sites of Action and Biological Effects; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Carlini, E.A.; Maia, L.O. Plant and Fungal Hallucinogens as Toxic and Therapeutic Agents. In Plant Toxins. Toxinology; Gopalakrishnakone, P., Carlini, C., Ligabue-Braun, R., Eds.; Springer: Dordrecht, Holland, 2017; pp. 37–80. [Google Scholar] [CrossRef]
- Bhatt, I.D.; Dauthal, P.; Rawat, S.; Gaira, K.S.; Jugran, A.; Rawal, R.S.; Dhar, U. Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Sci. Hortic. 2012, 136, 61–68. [Google Scholar] [CrossRef]
- Sangeeta, P.S.; Mathela, C.S.; Chopra, K. Elucidation of possible mechanism of analgesic action of Valeriana wallichii DC chemotype (patchouli alcohol) in experimental animal model. Indian J. Exp. Biol. 2010, 48, 289–293. [Google Scholar]
- Purnima, B.M.; Kothiyal, P. A review article on phytochemistry and pharmacological profiles of Nardostachys jatamansi DC-medicinal herb. J. Pharm. Phytochem. 2015, 3, 102–106. [Google Scholar]
- Sahu, R.; Dhongade, H.J.; Pandey, A.; Sahu, P.; Sahu, V.; Patel, D.; Kashyap, P. Medicinal Properties of Nardostachys jatamansi (A Review). Orient J. Chem. 2016, 32, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Choedon, T.; Kumar, V. Medicinal Plants used in the Practice of Tibetan Medicine. In Recent Progress in Medicinal Plants; Govil, G.N., Ed.; Studium Press: New Delhi, India, 2012; Volume 34, pp. 387–405. [Google Scholar]
- Nandhini, S.; Narayanan, K.B.; Ilango, K. Valeriana officinalis: A review of its traditional uses, phytochemistry and pharmacology. Asian J. Pharm. Clin. Res. 2018, 11, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Rather, A.M.; Nawchoo, I.A.; Ganie, A.H.; Singh, H.; Dutt, B.; Wani, A.A. Bioactive Compounds & Medicinal Properties of Valeriana jatamansi Jones—A review. Life Sci. J. 2012, 9, 952–955. [Google Scholar]
- Jha, S.V.; Bhagwat, A.M.; Pandita, N.S. Pharmacognostic and Phytochemical studies on the rhizome of Nardostachys jatamansi DC. using different extracts. Pharmacogn. J. 2012, 4, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Dubey, P.; Srivastava, S.; Rawat, A.K.S. Botanical standardization of the Jatamansi, their substitute and adulterant species. IJTK 2011, 10, 599–603. [Google Scholar]
- Dhimana, N.; Bhattacharya, A. Nardostachys jatamansi (D.Don) DC.-Challenges and opportunities of harnessing the untapped medicinal plant from the Himalayas. J. Ethnopharmacol. 2020, 112211. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Jung, H.Y.; Nam, S.Y.; Kim, J.W.; Choi, J.H.; Kwak, Y.G.; Yoo, M.; Lee, S.; Yoon, Y.S.; Hwang, I.K. Valeriana officinalis Extracts Ameliorate Neuronal Damage by Suppressing Lipid Peroxidation in the Gerbil Hippocampus Following Transient Cerebral Ischemia. J. Med. Food 2015, 18, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadley, S.; Petry, J.J. Valerian. Am. Fam. Physician 2003, 67, 1755–1758. [Google Scholar] [PubMed]
- Jung, H.Y.; Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, J.W.; Choi, J.H.; Kwak, Y.G.; Yoon, Y.S.; Hwang, I.K. Valeriana officinalis root extract suppresses physical stress by electric shock and psychological stress by nociceptive stimulation-evoked responses by decreasing the ratio of monoamine neurotransmitters to their metabolites. BMC Complement. Altern. Med. 2014, 14, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raina, A.P.; Negi, K.S. Essential Oil Composition of Valeriana jatamansi Jones from Himalayan Regions of India. Indian J. Pharm. Sci. 2015, 77, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Y.; Guo, Y.; Guo, P.; Yamakuni, T.; Ohizumi, Y. Isolation, Structural Elucidation, and Neuroprotective Effects of Iridoids from Valeriana jatamansi. Biosci. Biotechnol. Biochem. 2012, 76, 1401–1403. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.; Singh, S.K.; Nag, A.; Ahuja, P.S. Genetic Structure of Indian Valerian (Valeriana jatamansi) Populations in Western Himalaya Revealed by AFLP. Biochem. Genet. 2011, 49, 674–698. [Google Scholar] [CrossRef]
- Razack, S.; Kandikattu, H.K.; Venuprasad, M.P.; Amruta, N.; Khanum, F.; Chuttani, K.; Mishra, A.K. Anxiolytic actions of Nardostachys jatamansi via GABA benzodiazepine channel complex mechanism and its biodistribution studies. Metab. Brain Dis. 2018, 33, 1533–1549. [Google Scholar] [CrossRef]
- Kamini, G.; Raina, R. Review of Nardostachys grandiflora: An Important Endangered Medicinal and Aromatic Plant of Western Himalaya. For. Prod. J. 2013, 63, 67–71. [Google Scholar] [CrossRef]
- Prakash, V. Indian Valerianaceae: A Monograph On Medicinally Important Family; Scientific Publishers: Jodphur, India, 1999. [Google Scholar]
- Taibi, D.M.; Landis, C.A.; Petry, H.; Vitiello, M.V. A systematic review of valerian as a sleep aid: Safe but not effective. Sleep Med. Rev. 2007, 11, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Verma, R.K.; Padalia, R.C.; Chauhan, A.; Singh, A.; Singh, H.P. Chemical Diversity in the Essential Oil of Indian Valerian (Valeriana jatamansi Jones). Chem. Biodivers. 2011, 8, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Matsumoto, T.; Motomura, E.; Shiroyama, T. The Sleep-Enhancing Effect of Valerian Inhalation and Sleep-Shortening Effect of Lemon Inhalation. Chem. Senses 2006, 31, 731–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saroya, A.S.; Singh, J. Neuropharmacology of Nardostachys jatamansi DC. In Pharmacotherapeutic Potential of Natural Products in Neurological Disorders; Springer: Singapore, 2018; pp. 167–174. [Google Scholar] [CrossRef]
- Houghton, P.J. The scientific basis for the reputed activity of Valerian. J. Pharm. Pharmacol. 1999, 51, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Samaneh, E.T.; Tayebeh, R.; Hassan, E.; Vahid, N. Composition of essential oils in subterranean organs of three species of Valeriana L. Nat. Prod. Res. 2010, 24, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Katoch, R.; Kapila, R.K. Genetic and biochemical diversity among Valeriana jatamansi populations from Himachal Pradesh. Sci. World J. 2015, 2015, 863913. [Google Scholar] [CrossRef] [Green Version]
- Valerian: The Genus Valeriana, 1st ed.; Houghton, P.J. (Ed.) Harwood Academic Publishers: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Rahfeld, B. Mikroskopischer Farbatlas Pflanzlicher Drogen; Springer Spektrum: Wiesbaden, Germany, 2017. [Google Scholar]
- American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines; Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. (Eds.) CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Jayaratne, R.K.; Hettiarachchi, P.L.; Abeysekera, A. A comparative pharmacognostic evaluation of anatomical and chemical characters of Nardostachys jatamansi (D.Don) DC. (Jatamansa) and Valeriana mooni Arn. Ex C.B. Clarke (Lanka Thuwarala). Sri Lankan J. Biol. 2017, 2, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Novellino, A.; Scelfo, B.; Palosaari, T.; Price, A.; Sobanski, T.; Shafer, T.J.; Johnstone, A.F.; Gross, G.W.; Gramowski, A.; Schroeder, O.; et al. Development of micro-electrode array based tests for neurotoxicity: Assessment of interlaboratory reproducibility with neuroactive chemicals. Front. Neuroeng. 2011, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Maiwulanjiang, M.; Bi, C.W.; Lee, P.S.; Xin, G.; Miernisha, A.; Lau, K.M.; Xiong, A.; Li, N.; Dong, T.T.; Aisa, H.A.; et al. The volatile oil of Nardostachyos Radix et Rhizoma induces endothelial nitric oxide synthase activity in HUVEC cells. PLoS ONE 2015, 10, e0116761. [Google Scholar] [CrossRef]
- Costa, R.; De Fina, M.R.; Valentino, M.R.; Dugo, P.; Mondello, L. Reliable Identification of Terpenoids and Related compounds by using Linear Retention Indices Interactively with Mass Spectrometry Search. Nat. Prod. Commun. 2007, 2, 413–418. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Nautiyal, M.C.; Figueredo, G.; Rana, V.S. Effect of Post Harvest Drying Methods on the Essential Oil Composition of Nardostachys jatamansi DC. JEOBP 2017, 20, 1090–1096. [Google Scholar] [CrossRef]
- Safaralie, A.; Fatemi, S.; Sefidkon, F. Essential oil composition of Valeriana officinalis L. roots cultivated in Iran. Comparative analysis between supercritical CO2 extraction and hydrodistillation. J. Chromatogr. A 2008, 1180, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Liu, H.; Zhou, L.; Liu, Z.; Wang, J.; Han, J.; Yu, Z.; Yang, F. Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules 2010, 15, 6411–6422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Ragusa, S.; Monforte, M.T.; D’Angelo, V.; Circosta, C. Phytochemical analysis and evaluation of antioxidant and anti-acetylcholinesterase activities of Euphorbia dendroides L. (Euphorbiaceae) latex. Plant Biosyst. 2019, 153, 498–505. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Houghton, P.J. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother. Res. 2007, 21, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Ran, X.H.; Chen, R.; Luo, H.R.; Ma, Q.Y.; Liu, Y.Q.; Hu, J.M.; Huang, S.Z.; Jiang, H.Z.; Chen, Z.Q.; et al. Sesquiterpenoids and lignans from the roots of Valeriana officinalis L. Chem. Biodivers 2011, 8, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Subramanian, S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS ONE 2014, 9, e86804. [Google Scholar] [CrossRef]
- Dong, F.W.; Yang, L.; Wu, Z.K.; Gao, W.; Zi, C.T.; Yang, D.; Luo, H.R.; Zhou, J.; Hu, J.M. Iridoids and sesquiterpenoids from the roots of Valeriana jatamansi Jones. Fitoterapia 2015, 102, 27–34. [Google Scholar] [CrossRef]
- Chen, H.W.; He, X.H.; Yuan, R.; Wei, B.J.; Chen, Z.; Dong, J.X.; Wang, J. Sesquiterpenes and a monoterpenoid with acetylcholinesterase (AChE) inhibitory activity from Valeriana officinalis var. latiofolia in vitro and in vivo. Fitoterapia 2016, 110, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Ahmad, M.; Zafar, K.S.; Ahmad, A.S.; Islam, F. Protective effect of Nardostachys jatamansi in rat cerebral ischemia. Pharmacol. Biochem. Behav. 2003, 74, 481–486. [Google Scholar] [CrossRef]
- Anonymous. The Wealth of India; Council for Scientific and Industrial Research: New Delhi, India, 1992; Volume 3. [Google Scholar]
- Khuda, F.; Iqbal, Z.; Zakiullah, K.A.; Shah, Y.; Khan, A. Report: Screening of selected medicinal plants for their enzyme inhibitory potential—A validation of their ethnopharmacological uses. Pak. J. Pharm. Sci. 2014, 27, 593–596. [Google Scholar] [PubMed]
- Park, I.K. Fumigant toxicity of Oriental sweetgum (Liquidambar orientalis) and valerian (Valeriana wallichii) essential oils and their components, including their acetylcholinesterase inhibitory activity, against Japanese termites (Reticulitermes speratus). Molecules 2014, 19, 12547–12558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramowski, A.; Jügelt, K.; Stüwe, S.; Schulze, R.; McGregor, G.P.; Wartenberg-Demand, A.; Loock, J.; Schröder, O.; Weiss, D.G. Functional screening of traditional antidepressants with primary cortical neuronal networks grown on multielectrode neurochips. Eur. J. Neurosci. 2006, 24, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Polunin, O.; Stainton, A. Flowers of the Himalaya; Oxford University Press: New Delhi, India, 1984; ISBN 0195641876. [Google Scholar]
- Joshi, K.K.; Joshi, S.D. Medicinal and Aromatic Plants Used in Nepal, Tibet and Trans-Himalayan Region; AuthorHouse: Bloomington, IN, USA, 2006; ISBN 1420865846. [Google Scholar]
- Plants of the World Online. Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 30 April 2020).
- Doyle, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; NATO ASI Series (Series H: Cell Biology); Hewitt, G.M., Johnston, A.W.B., Young, J.P.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 57, pp. 283–293. [Google Scholar]
- Frigerio, J.; Gorini, T.; Galimberti, A.; Bruni, I.; Tommasi, N.; Mezzasalma, V.; Labra, M. DNA barcoding to trace Medicinal and Aromatic Plants from the field to the food supplement. J. Appl. Bot Food Qual. 2019, 92, 33–38. [Google Scholar] [CrossRef]
- Frigerio, J.; Pellesi, R.; Mezzasalma, V.; De Mattia, F.; Galimberti, A.; Lambertini, F.; Suman, M.; Zanardi, S.; Leporati, A.; Labra, M. Development of a DNA Barcoding-Like Approach to Detect Mustard Allergens in Wheat Flours. Genes 2019, 10, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, I.; Galimberti, A.; Caridi, L.; Scaccabarozzi, D.; De Mattia, F.; Casiraghi, M.; Labra, M. A DNA barcoding approach to identify plant species in multiflower honey. Food Chem. 2015, 170, 308–315. [Google Scholar] [CrossRef]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Caputo, L.; Cornara, L.; Bazzicalupo, M.; De Francesco, C.; De Feo, V.; Trombetta, D.; Smeriglio, A. Chemical Composition and Biological Activities of Essential Oils from Peels of Three Citrus Species. Molecules 2020, 25, 1890. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectroscopy, 4.1 ed.; Allured Publishing: Carol Stream, IL, USA, 2017; pp. 1–809. [Google Scholar]
- NIST 08 Mass Spectral Library (NIST/EPA/NIH). 2008. Available online: www.nist.gov (accessed on 1 March 2020).






| Declared Species | Resulted Species | Origin | Collection Year | Accession Number |
|---|---|---|---|---|
| V. officinalis | V. officinalis | China | 2019 | LR861814 |
| V. jatamansi | V. jatamansi | Darchula District, Nepal | 2019 | LR861815 |
| N. jatamansi | N. jatamansi | Darchula District, Nepal | 2019 | LR861816 |
| Compound | V. officinalis | V. jatamansi | N. jatamansi | KI a | Identification b |
|---|---|---|---|---|---|
| 2-Acetylfuran | - | 0.50 ± 0.03 | 912 | 1,2 | |
| Tricyclene | 0.14 ± 0.01 | - | t | 926 | 1,2 |
| α-Thujene | 0.03 ± 0.00 | - | 0.06 ± 0.00 | 930 | 1,2 |
| α-Pinene | 3.39 ± 0.15 | 0.20 ± 0.01 | 1.59 ± 0.05 | 939 | 1,2,3 |
| 3-methyl-Valeric acid | - | 0.09 ± 0.00 | - | 945 | 1,2 |
| α-Fenchene | - | - | 0.01 ± 0.00 | 952 | 1,2 |
| Camphene | 13.85 ± 0.88 | 0.29 ± 0.01 | 0.03 ± 0.00 | 954 | 1,2,3 |
| Sabinene | 0.16 ± 0.01 | - | - | 975 | 1,2 |
| β-Thujene | - | - | 0.03 ± 0.00 | 976 | 1,2 |
| β-Pinene | 2.76 ± 0.12 | - | 2.73 ± 0.13 | 979 | 1,2,3 |
| α-Phellandrene | - | - | 0.01 ± 0.00 | 1002 | 1,2 |
| α-Terpinene | 0.02 ± 0.00 | - | 0.04 ± 0.00 | 1017 | 1,2 |
| p-Cymene | 0.32 ± 0.01 | 0.11 ± 0.01 | 0.08 ±0.00 | 1023 | 1,2 |
| Limonene | 1.50 ± 0.04 | 0.07 ± 0.00 | 0.21 ± 0.01 | 1029 | 1,2,3 |
| 1,8-Cineole | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.18 ± 0.01 | 1031 | 1,2,3 |
| γ-Terpinene | 0.15 ± 0.01 | - | 0.07 ± 0.00 | 1059 | 1,2 |
| Terpinolene | 0.05 ± 0.00 | - | 0.05 ± 0.00 | 1088 | 1,2 |
| 2-Nonanone | - | 0.01 ± 0.00 | 1090 | 1,2 | |
| Isoamylvalerate | - | - | 0.01 ± 0.00 | 1081 | 1,2 |
| Linalool | - | - | 0.02 ± 0.00 | 1096 | 1,2,3 |
| Fenchol | - | - | 0.01 ± 0.00 | 1116 | 1,2 |
| Camphor | 0.07 ± 0.00 | - | - | 1146 | 1,2 |
| Borneol | 0.87 ± 0.03 | 0.17 ± 0.01 | 0.02 ± 0.00 | 1169 | 1,2,3 |
| cis-3-Pinanone | - | - | 0.03 ± 0.00 | 1174 | 1,2 |
| Terpinen-4-ol | 0.18 ± 0.01 | - | 0.14 ± 0.01 | 1177 | 1,2,3 |
| α-Terpineol | 0.13 ± 0.01 | - | 0.15 ± 0.01 | 1188 | 1,2 |
| Myrtenol | 0.28 ± 0.02 | - | 0.09 ± 0.00 | 1195 | 1,2 |
| Citronellol | 0.61 ± 0.03 | - | - | 1225 | 1,2,3 |
| Thymol, methyl ether | 0.30 ± 0.01 | 0.05 ± 0.00 | 0.11 ± 0.01 | 1235 | 1,2 |
| Carvacrol, methyl ether | - | - | 0.36 ± 0.02 | 1244 | 1,2 |
| Furfurylvalerate | - | 0.14 ± 0.01 | 1271 | 1,2 | |
| Bornyl acetate | 46.90 ± 1.24 | 0.42 ± 0.02 | 0.02 ± 0.00 | 1285 | 1,2,3 |
| Undecan-2-one | - | - | 0.69 ± 0.03 | 1294 | 1,2 |
| 1,2,3-Trimethylindene | - | - | 0.06 ± 0.00 | 1299 | 1,2 |
| Mirtenyl acetate | 3.94 ± 0.22 | - | 0.40 ± 0.02 | 1326 | 1,2 |
| δ-Elemene | - | - | 0.19 ± 0.01 | 1338 | 1,2 |
| α-Cubebene | - | - | 0.09 ± 0.00 | 1351 | 1,2 |
| Cyclosativene | - | - | 0.03 ± 0.00 | 1371 | 1,2 |
| α-Copaene | - | - | 1.90 ± 0.14 | 1376 | 1,2 |
| β-Patchoulene | - | 6.02 ± 0.32 | 0.90 ± 0.04 | 1380 | 1,2 |
| β-Cubebene | - | - | 0.35 ± 0.02 | 1386 | 1,2 |
| β-Elemene | 1.30 ± 0.05 | 0.55 ± 0.02 | 1.54 ± 0.06 | 1389 | 1,2 |
| Cyperene | - | 3.42 ± 0.24 | 1398 | 1,2 | |
| β-Longipinene | - | 1.11 ± 0.04 | - | 1400 | 1,2 |
| α-Gurjunene | 1.13 ± 0.04 | - | - | 1409 | 1,2 |
| β-Maaliene | - | - | 2.07 ± 0.18 | 1415 | 1,2 |
| α-Santalene | - | 3.35 ± 0.18 | - | 1417 | 1,2 |
| 9-Aristolene | - | - | 1.42 ± 0.05 | 1418 | 1,2 |
| (E)-β-Caryophyllene | 0.30 ± 0.02 | 0.37 ± 0.02 | 2.94 ± 0.22 | 1419 | 1,2,3 |
| Calarene | 0.17 ± 0.01 | 10.57 ± 0.05 | 8.22 ± 0.44 | 1433 | 1,2 |
| α-trans-Bergamotene | - | 0.93 ± 0.04 | - | 1434 | 1,2 |
| α-Guaiene | - | 2.58 ± 0.14 | 0.05 ± 0.00 | 1439 | 1,2 |
| Aromadendrene | - | 6.43 ± 0.35 | 4.17 ± 0.26 | 1441 | 1,2,3 |
| Seychellene | - | 4.27 ± 0.28 | 6.90 ± 0.38 | 1446 | 1,2 |
| α-Humulene | 0.32 ± 0.01 | 1.29 ± 0.05 | 0.82 ± 0.04 | 1453 | 1,2,3 |
| α-Patchoulene | - | 3.35 ± 0.16 | 0.51 ± 0.02 | 1456 | 1,2 |
| allo-Aromadendrene | 1.21 ± 0.04 | - | - | 1458 | 1,2 |
| γ-Gurjunene | - | 11.88 ± 0.48 | 6.36 ± 0.25 | 1477 | 1,2 |
| ar-Curcumene | - | - | 1.07 ± 0.03 | 1479 | 1,2 |
| Germacrene D | 0.05 ± 0.00 | - | 0.50 ± 0.02 | 1481 | 1,2 |
| (E)-β-Ionone | 0.14 ± 0.01 | - | 0.19 ± 0.01 | 1488 | 1,2 |
| β-Selinene | - | - | 2.55 ± 0.11 | 1490 | 1,2 |
| Valencene | - | 0.19 ± 0.01 | 8.05 ± 0.37 | 1496 | 1,2 |
| Bicyclogermacrene | 0.55 ± 0.02 | - | - | 1500 | 1,2 |
| γ-Patchoulene | - | 0.76 ± 0.03 | - | 1502 | 1,2 |
| α-Bulnesene | - | - | 0.26 ± 0.02 | 1510 | 1,2 |
| γ-Cadinene | - | - | 0.07 ± 0.00 | 1513 | 1,2 |
| δ-Guaiene | - | 8.53 ± 0.24 | - | 1516 | 1,2 |
| α-Maaliene | - | - | 1.27 ± 0.06 | 1522 | 1,2 |
| δ-Cadinene | - | 0.07 ± 0.00 | - | 1523 | 1,2 |
| Zonarene | - | - | 1.77 ± 0.07 | 1529 | 1,2 |
| Kessane | - | 2.51 ± 0.12 | - | 1530 | 1,2 |
| α-Cadinene | 0.50 ± 0.03 | 0.35 ±0.01 | 0.86 ± 0.04 | 1538 | 1,2 |
| Elemol | - | 1.08 ± 0.03 | - | 1549 | 1,2 |
| Norpatchoulenol | - | 8.06 ± 0.25 | - | 1555 | 1,2 |
| GermacreneB | 0.31 ± 0.01 | - | - | 1561 | 1,2 |
| Maaliol | - | 17.43 ± 0.56 | - | 1567 | 1,2 |
| α-Vatirenene | - | - | 3.07 ± 0.21 | 1570 | 1,2 |
| Spathulenol | 0.84 ± 0.03 | 1.32 ± 0.04 | 0.52 ± 0.02 | 1577 | 1,2 |
| Spirojatamol | - | - | 3.49 ± 0.18 | 1585 | 1,2 |
| Globulol | 0.84 ± 0.04 | 0.45 ± 0.02 | 0.48 ± 0.03 | 1590 | 1,2 |
| Viridiflorol | - | 0.16 ± 0.01 | 1.88 ± 0.08 | 1592 | 1,2 |
| Guaiol | 1.11 ± 0.02 | 0.57 ± 0.02 | - | 1600 | 1,2,3 |
| Ledol | - | - | 0.11 ± 0.01 | 1602 | 1,2 |
| Hinesol | 0.28 ± 0.01 | - | - | 1641 | 1,2 |
| τ-Muurolol | - | - | 1.24 ± 0.10 | 1642 | 1,2 |
| Valeranol | 0.56 ± 0.02 | 0.97 ± 0.03 | - | 1652 | 1,2 |
| α-Cadinol | - | - | 2.24 ± 0.20 | 1654 | 1,2 |
| Patchoulol | - | 0.41 ± 0.02 | - | 1658 | 1,2 |
| Jatamansone | - | - | 13.96 ± 0.74 | 1675 | 1,2 |
| Valeranal | - | - | 4.39 ± 0.25 | 1706 | 1,2 |
| Aristolone | - | - | 2.44 ± 0.13 | 1763 | 1,2 |
| Nootkatone | - | - | 0.44 ± 0.02 | 1806 | 1,2 |
| trans-Valerenyl acetate | 13.18 ± 0.55 | - | - | 1867 | 1,2 |
| cis-Valerenylisovalerate | 1.53 ± 0.04 | - | - | 2052 | 1,2 |
| Total | 100.00 | 100.00 | 100.00 | ||
| Monoterpene hydrocarbons | 23.70 | 0.67 | 4.83 | ||
| Oxygenated monoterpenes | 53.29 | 0.19 | 0.53 | ||
| Sesquiterpene hydrocarbons | 4.08 | 67.23 | 61.59 | ||
| Oxygenated sesquiterpenes | 4.44 | 30.45 | 23.93 | ||
| Others | 14.71 | 1.46 | 9.123 |
| Compound | V. officinalis | V. jatamansi | N. jatamansi |
|---|---|---|---|
| α-Thujene | + | - | + |
| α-Pinene | + | + | + |
| 3-methyl-Valeric acid | - | + | - |
| Camphene | + | + | - |
| β-Pinene | + | - | - |
| p-Cymene | + | + | - |
| Limonene | + | + | - |
| 1,8-Cineole | + | - | - |
| γ-Terpinene | + | - | - |
| Linalool | - | - | + |
| Borneol | + | + | - |
| Citronellol | + | - | - |
| Thymol, methyl ether | - | + | + |
| Carvacrol, methyl ether | - | - | + |
| Bornyl acetate | + | + | - |
| Undecan-2-one | - | - | + |
| Mirtenyl acetate | + | - | + |
| δ-Elemene | - | - | + |
| α-Cubebene | - | - | + |
| Cyclosativene | - | - | + |
| α-Copaene | - | - | + |
| β-Patchoulene | - | + | + |
| β-Cubebene | - | - | + |
| β-Elemene | + | + | - |
| Cyperene | - | - | + |
| β-Longipinene | - | + | - |
| α-Gurjunene | + | - | + |
| β-Maaliene | - | - | + |
| α-Santalene | - | + | - |
| 9-Aristolene | - | - | + |
| (E)-β-Caryophyllene | + | + | - |
| Calarene | - | + | + |
| α-Guaiene | - | + | + |
| Aromadendrene | - | + | - |
| Seychellene | - | + | + |
| α-Humulene | + | + | + |
| α-Patchoulene | - | + | - |
| allo-Aromadendrene | + | - | - |
| γ-Gurjunene | - | + | - |
| Germacrene D | + | - | - |
| (E)-β-Ionone | + | - | + |
| β-Selinene | - | - | + |
| Valencene | - | + | + |
| Bicyclogermacrene | + | - | - |
| γ-Patchoulene | - | + | - |
| α-Bulnesene | - | - | + |
| γ-Cadinene | - | - | + |
| δ-Guaiene | - | + | - |
| α-Maaliene | - | - | + |
| δ-Cadinene | - | + | + |
| Zonarene | - | - | + |
| Kessane | - | + | - |
| α-Cadinene | - | + | - |
| Germacrene B | + | - | - |
| Maaliol | - | + | - |
| Spathulenol | + | + | + |
| Spirojatamol | - | - | + |
| Globulol | + | - | + |
| Viridiflorol | - | + | + |
| Guaiol | + | + | - |
| Ledol | - | - | + |
| Hinesol | + | - | - |
| τ-Muurolol | - | - | + |
| Valerenol | + | - | - |
| α-Cadinol | - | - | + |
| Patchoulol | - | + | + |
| Jatamansone | - | - | + |
| Valeranal | - | - | + |
| Aristolone | - | - | + |
| trans-Valerenyl acetate | + | - | - |
| cis-Valerenylisovalerate | + | - | - |
| References | [20,31,32] | [3,15,18,22] | [28,29,30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornara, L.; Ambu, G.; Trombetta, D.; Denaro, M.; Alloisio, S.; Frigerio, J.; Labra, M.; Ghimire, G.; Valussi, M.; Smeriglio, A. Comparative and Functional Screening of Three Species Traditionally used as Antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D.Don) DC. Plants 2020, 9, 994. https://doi.org/10.3390/plants9080994
Cornara L, Ambu G, Trombetta D, Denaro M, Alloisio S, Frigerio J, Labra M, Ghimire G, Valussi M, Smeriglio A. Comparative and Functional Screening of Three Species Traditionally used as Antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D.Don) DC. Plants. 2020; 9(8):994. https://doi.org/10.3390/plants9080994
Chicago/Turabian StyleCornara, Laura, Gabriele Ambu, Domenico Trombetta, Marcella Denaro, Susanna Alloisio, Jessica Frigerio, Massimo Labra, Govinda Ghimire, Marco Valussi, and Antonella Smeriglio. 2020. "Comparative and Functional Screening of Three Species Traditionally used as Antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D.Don) DC." Plants 9, no. 8: 994. https://doi.org/10.3390/plants9080994
APA StyleCornara, L., Ambu, G., Trombetta, D., Denaro, M., Alloisio, S., Frigerio, J., Labra, M., Ghimire, G., Valussi, M., & Smeriglio, A. (2020). Comparative and Functional Screening of Three Species Traditionally used as Antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D.Don) DC. Plants, 9(8), 994. https://doi.org/10.3390/plants9080994