The Promise of Turning Induced Deformation Process for Synthesizing Magnesium Based Materials with Superior Mechanical Response
Abstract
:1. Introduction
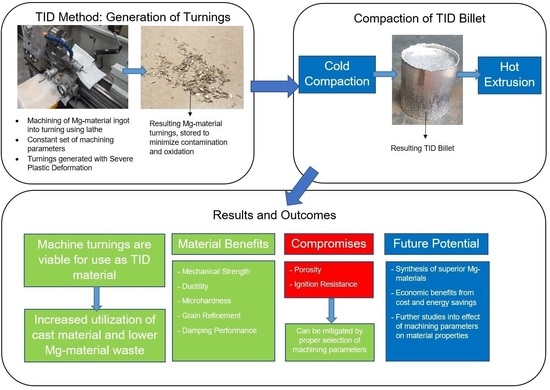
2. TID Methodology
3. Results
3.1. TID Effect on Porosity
3.2. TID Effect on Ignition Resistance
3.3. TID Effect on Damping Performance
3.4. TID Effect on Mechanical Strength
3.5. TID Effect on Microhardness
3.6. TID Effect on Grain Size
4. Conclusions
- Turnings generated from machining were viable for direct utilization in further processing to billets, thus increasing the proportion of material utilized following primary processing (casting) relative to conventional processing methods where material beyond specified dimensions is normally machined off and discarded. In addition, this was achieved without use of sintering, resulting in a processing method that confers energy savings as well as reducing Mg-material waste and requirements for a given production volume.
- The TID method consistently produced materials possessing superior properties to that of conventionally processed materials with minimal compromises, opening up the path to synthesis of superior Mg materials. Notably, increased mechanical strength, ductility, microhardness, and damping performance were observed with only minimal compromises in porosity and ignition resistance.
- Machining parameters such as higher DOCs and lower cutting speeds have produced Mg materials with the most desirable properties including low porosity, higher ignition resistance, finer grains, higher mechanical strength and microhardness, as well as damping capacities without significant compromises in ductility, providing a basis for further studies on parameter adjustment and effects on outcomes of TID-processed materials.
Author Contributions
Funding
Conflicts of Interest
References
- Cao, H.; Pistidda, C.; Riglos, M.V.C.; Chaudhary, A.-L.; Capurso, G.; Tseng, J.-C.; Puszkiel, J.; Wharmby, M.T.; Gemming, T.; Chen, P.; et al. Conversion of magnesium waste into a complex magnesium hydride system: Mg(NH2)2–LiH. Sustain. Energy Fuels 2020, 4, 1915–1923. [Google Scholar] [CrossRef]
- Maier, P.; Hort, N. Magnesium Alloys for Biomedical Applications. Metals 2020, 10, 1328. [Google Scholar] [CrossRef]
- Akyuz, B. Machinability of Magnesium and Its Alloys. TOJSAT Online J. Sci. Technol. 2011, 1, 31–38. [Google Scholar]
- Blawert, N.H.C.; Kainer, K.U. Automotive Application of Magnesium and Its Alloys. Trans. Indian Inst. Met. 2004, 57, 397–408. [Google Scholar]
- Lapovok, R.Y.; Thomson, P.F. Production of dense rod from magnesium swarf for re-melting. Magnes. Technol. 2004, 1, 149–154. [Google Scholar]
- Bell, R.W.N.; Parker, D.; Baker, K.; Lee, P.; Magnesium Recycling in the EU. Oakdene Hollins. 7 June 2017. Available online: https://cdn.ymaws.com/www.intlmag.org/resource/resmgr/sustainability/FullRprt_EU-Mg-recycling_201.pdf (accessed on 13 August 2021).
- Ditze; Scharf, C. Recycling of Magnesium Alloys. In Magnesium—Alloys and Technology; Wiley Online Books: Hoboken, NJ, USA, 2003; pp. 254–278. [Google Scholar]
- Survey, U.S.G. Mineral Commodity Summaries 2021. In Mineral Commodity Summaries; Reston, V.A., Ed.; Report; 2021. Available online: http://pubs.er.usgs.gov/publication/mcs2021 (accessed on 13 August 2021).
- Shamsudin, S.; Lajis, M.A.; Zhong, Z.W. Solid-state recycling of light metals: A review. Adv. Mech. Eng. 2016, 8, 1687814016661921. [Google Scholar] [CrossRef] [Green Version]
- Powell IV, A.C.; Pati, S.; Derezinski, S.; Strauss, J.; Pal, U.; Zink, P.; Guan, X. Efficient One-Step Electrolytic Recycling of Low-Grade and Post-Consumer Magnesium Scrap. Metal Oxygen Separation Technologies, Inc., 19 July 2012. Available online: https://www.osti.gov/servlets/purl/1046980 (accessed on 13 August 2021).
- Galanty, M.; Kazanowski, P.; Kansuwan, P.; Misiolek, W.Z. Consolidation of metal powders during the extrusion process. J. Mater. Process. Technol. 2002, 125–126, 491–496. [Google Scholar] [CrossRef]
- Chiba, R.; Yoshimura, M. Solid-state recycling of aluminium alloy swarf into c-channel by hot extrusion. J. Manuf. Process. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Fan, Z. A physical approach to the direct recycling of Mg-alloy scrap by the rheo-diecasting process. Mater. Sci. Eng. A 2008, 472, 251–257. [Google Scholar] [CrossRef]
- Chino, Y.; Kobata, M.; Shimojima, K.; Hosokawa, H.; Yamada, Y.; Iwasaki, H.; Mabuchi, M. Blow Forming of Mg Alloy Recycled by Solid-State Recycling. Mater. Trans. 2004, 45, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Chino, Y.; Jae-Seol, L.; Nakaura, Y.; Ohori, K.; Mabuchi, M. Mechanical Properties of Mg-Al-Ca Alloy Recycled by Solid-State Recycling. Mater. Trans. 2005, 46, 2592–2595. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, M.; Kubota, K.; Higashi, K. New Recycling Process by Extrusion for Machined Chips of AZ91 Magnesium and Mechanical Properties of Extruded Bars. Mater. Trans. JIM 1995, 36, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ji, Z.; Zhang, T. Microstructure and mechanical properties of AZ31B magnesium alloy recycled by solid-state process from different size chips. J. Mater. Process. Technol. 2009, 209, 5319–5324. [Google Scholar] [CrossRef]
- Tekumalla, S.; Ajjarapu, M.; Gupta, M. A Novel Turning-Induced-Deformation Based Technique to Process Magnesium Alloys. Metals 2019, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Ghadbeigi, H.; Bradbury, S.R.; Pinna, C.; Yates, J.R. Determination of micro-scale plastic strain caused by orthogonal cutting. Int. J. Mach. Tools Manuf. 2008, 48, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Lapovok, R.; Estrin, Y. 4—Superplasticity in magnesium alloys by severe plastic deformation. In Advances in Wrought Magnesium Alloys; Bettles, C., Barnett, M., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 144–185. [Google Scholar]
- Yuan, Y.; Ma, A.; Jiang, J.; Lu, F.; Jian, W.; Song, D.; Zhu, Y.T. Optimizing the strength and ductility of AZ91 Mg alloy by ECAP and subsequent aging. Mater. Sci. Eng. A 2013, 588, 329–334. [Google Scholar] [CrossRef]
- Al-Zubaydi, A.S.J.; Zhilyaev, A.P.; Wang, S.C.; Reed, P.A.S. Superplastic behaviour of AZ91 magnesium alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2015, 637, 1–11. [Google Scholar] [CrossRef]
- Rao, K.; Suresh, K.; Prasad, Y.; Hort, N.; Gupta, M. Enhancement of Strength and Hot Workability of AZX312 Magnesium Alloy by Disintegrated Melt Deposition (DMD) Processing in Contrast to Permanent Mold Casting. Metals 2018, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Johanes, M.; Tekumalla, S.; Gupta, M. Fe3O4 Nanoparticle-Reinforced Magnesium Nanocomposites Processed via Disintegrated Melt Deposition and Turning-Induced Deformation Techniques. Metals 2019, 9, 1225. [Google Scholar] [CrossRef] [Green Version]
- Tekumalla, S.; Gupta, N.; Gupta, M. Influence of turning speed on the microstructure and properties of magnesium ZK60 alloy pre-processed via turning-induced-deformation. J. Alloy. Compd. 2020, 831, 154840. [Google Scholar] [CrossRef]
- Guo, Y.; Salahshoor, M. Process mechanics and surface integrity by high-speed dry milling of biodegradable magnesium–calcium implant alloys. CIRP Ann. 2010, 59, 151–154. [Google Scholar] [CrossRef]
- Gugulothu, B.; Kumsa, D.K.; Kassa, M.B. Effect of process parameters on centre lathe of EN8 steel in turning process. Mater. Today Proc. 2020, 46, 228–233. [Google Scholar] [CrossRef]
- Tekumalla, S.; Chun, L.S.; Gupta, M. Preprocessing of powder to enhance mechanical and thermal response of bulk magnesium. Met. Powder Rep. 2019, 74, 137–140. [Google Scholar] [CrossRef]
- Atlas Foundry Company. Mechanical Properties of Gray Iron—Damping Capacity. Available online: http://www.atlasfdry.com/grayiron-damping.htm (accessed on 6 August 2021).
- Li, Q.; Jiang, G.; Dong, J.; Hou, J.; He, G. Damping behavior and energy absorption capability of porous magnesium. J. Alloy. Compd. 2016, 680, 522–530. [Google Scholar] [CrossRef]
- Xie, Z.-K.; Tane, M.; Hyun, S.-K.; Okuda, Y.; Nakajima, H. Vibration–damping capacity of lotus-type porous magnesium. Mater. Sci. Eng. A 2006, 417, 129–133. [Google Scholar] [CrossRef]
- Li, B.; Lavernia, E.J. Spray Forming of MMCs. In Comprehensive Composite Materials, 1st ed.; Kelly, A., Zweben, C., Eds.; Online; Elsevier: Amsterdam, The Netherlands, 2000; Volume 3, pp. 617–653. [Google Scholar]
- Gupta, M.; Wong, W.L.E. Magnesium-based nanocomposites: Lightweight materials of the future. Mater. Charact. 2015, 105, 30–46. [Google Scholar] [CrossRef]
- Tekumalla, S.; Shabadi, R.; Yang, C.; Seetharaman, S.; Gupta, M. Strengthening due to the in-situ evolution of ß1′ Mg-Zn rich phase in a ZnO nanoparticles introduced Mg-Y alloy. Scr. Mater. 2017, 133, 29–32. [Google Scholar] [CrossRef]
- Tekumalla, S.; Yang, C.; Seetharaman, S.; Wong, W.L.E.; Goh, C.S.; Shabadi, R.; Gupta, M. Enhancing overall static/dynamic/damping/ignition response of magnesium through the addition of lower amounts (<2%) of yttrium. J. Alloy. Compd. 2016, 689, 350–358. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.W.; Moon, B.G.; Kim, H.S.; Kim, Y.M.; Park, S.H. Influence of extrusion temperature on dynamic deformation behaviors and mechanical properties of Mg-8Al-0.5Zn-0.2Mn-0.3Ca-0.2Y alloy. J. Mater. Res. Technol. 2019, 8, 5254–5270. [Google Scholar] [CrossRef]
- Stanford, N. Micro-alloying Mg with Y, Ce, Gd and La for texture modification—A comparative study. Mater. Sci. Eng. A 2010, 527, 2669–2677. [Google Scholar] [CrossRef]
- Yu, H.; Xin, Y.; Wang, M.; Liu, Q. Hall-Petch relationship in Mg alloys: A review. J. Mater. Sci. Technol. 2018, 34, 248–256. [Google Scholar] [CrossRef]
- Yang, W.; Tekumalla, S.; Gupta, M. Cumulative Effect of Strength Enhancer—Lanthanum and Ductility Enhancer—Cerium on Mechanical Response of Magnesium. Metals 2017, 7, 241. [Google Scholar] [CrossRef] [Green Version]
- Brush Wellman, Inc. Grain Size and Material Strength. In Technical Tidbits; Brush Wellman, Inc.: Mayfield Heights, OH, USA, 2010. [Google Scholar]




| Material | Fabrication Method | Theoretical Density (g/cc) | Experimental Density (g/cc) | Porosity (%) |
|---|---|---|---|---|
| AZ91_AR | Conventional | 1.835 | 1.816 | 1.06 |
| AZ91_0.5DOC | TID (0.5 mm DOC) | 1.835 | 1.815 | 1.10 |
| AZ91_1DOC | TID (1.0 mm DOC) | 1.835 | 1.812 | 1.27 |
| AZ91_1.5DOC | TID (1.5 mm DOC) | 1.835 | 1.817 | 1.02 |
| Mg/1Fe3O4 DMD | Conventional | 1.77 | 1.76 ± 0.00 | 0.56 |
| Mg/2Fe3O4 DMD | Conventional | 1.81 | 1.78 ± 0.01 | 1.66 |
| Mg/3Fe3O4 DMD | Conventional | 1.84 | 1.79 ± 0.02 | 2.72 |
| Mg/1Fe3O4 TID | TID (one set of parameters) | 1.77 | 1.75 ± 0.01 | 1.13 |
| Mg/2Fe3O4 TID | TID (one set of parameters) | 1.81 | 1.76 ± 0.02 | 2.76 |
| Mg/3Fe3O4 TID | TID (one set of parameters) | 1.84 | 1.77 ± 0.02 | 3.81 |
| ZK60_AR | Conventional | 1.83 | 1.825 ± 0.002 | 0.26 |
| ZK60_HS | TID (high cutting speed) | 1.83 | 1.807 ± 0.025 | 1.23 |
| ZK60_MS | TID (medium cutting speed) | 1.83 | 1.816 ± 0.007 | 0.77 |
| ZK60_LS | TID (low cutting speed) | 1.83 | 1.822 ± 0.005 | 0.4 |
| Material | Fabrication Method | Ignition Temperature (°C) |
|---|---|---|
| Mg/1Fe3O4 DMD | Conventional | 630.6 |
| Mg/2Fe3O4 DMD | Conventional | 635.0 |
| Mg/3Fe3O4 DMD | Conventional | 635.2 |
| Mg/1Fe3O4 TID | TID (one set of parameters) | 625.4 |
| Mg/2Fe3O4 TID | TID (one set of parameters) | 633.1 |
| Mg/3Fe3O4 TID | TID (one set of parameters) | 632.2 |
| ZK60_AR | Conventional | 556 |
| ZK60_HS | TID (high cutting speed) | 521 |
| ZK60_MS | TID (medium cutting speed) | 528 |
| ZK60_LS | TID (low cutting speed) | 544 |
| Material | Fabrication Method | Damping Capacity (×10−6) | Elastic Modulus (GPa) |
|---|---|---|---|
| Mg/1Fe3O4 DMD | Conventional | 410 ± 48 | 49.58 |
| Mg/2Fe3O4 DMD | Conventional | 673 ± 179 | 46.92 |
| Mg/3Fe3O4 DMD | Conventional | 1303 ± 143 | 46.05 |
| Mg/1Fe3O4 TID | TID (one set of parameters) | 453 ± 27 | 48.70 |
| Mg/2Fe3O4 TID | TID (one set of parameters) | 690 ± 22 | 46.31 |
| Mg/3Fe3O4 TID | TID (one set of parameters) | 1735 ± 206 | 46.85 |
| ZK60_AR | Conventional | 99 | 47.78 ± 0.29 |
| ZK60_HS | TID (high cutting speed) | 138 | 45.21 ± 0.27 |
| ZK60_MS | TID (medium cutting speed) | 92 | 47.98 ± 0.29 |
| ZK60_LS | TID (low cutting speed) | 145 | 46.89 ± 0.28 |
| Material | Fabrication Method | 0.2% Yield Strength (MPa) | Failure Strain (%) |
|---|---|---|---|
| AZ91_AR | Conventional | 207 | 20.3 ± 1.3 |
| AZ91_0.5DOC | TID (0.5 mm DOC) | 275 | 21.5 ± 3.3 |
| AZ91_1DOC | TID (1.0 mm DOC) | 309 | 20 ± 2.0 |
| AZ91_1.5DOC | TID (1.5 mm DOC) | 375 | 19 ± 3.9 |
| Mg/1Fe3O4 DMD | Conventional | 75 | 15.8 |
| Mg/2Fe3O4 DMD | Conventional | 100 | 16.9 |
| Mg/3Fe3O4 DMD | Conventional | 76 | 18.9 |
| Mg/1Fe3O4 TID | TID (one set of parameters) | 99 | 18.2 |
| Mg/2Fe3O4 TID | TID (one set of parameters) | 111 | 18.4 |
| Mg/3Fe3O4 TID | TID (one set of parameters) | 91 | 19.1 |
| ZK60_AR | Conventional | 284 ± 3 | 20 ± 0.2 |
| ZK60_HS | TID (high cutting speed) | 257 ± 1 | 20 ± 1.4 |
| ZK60_MS | TID (medium cutting speed) | 299 ± 26 | 22 ± 1 |
| ZK60_LS | TID (low cutting speed) | 319 ± 3 | 21 ± 3 |
| Material | Fabrication Method | Microhardness (HV) |
|---|---|---|
| AZ91_AR | Conventional | 155 ± 7 |
| AZ91_0.5DOC | TID (0.5 mm DOC) | 157 ± 9 |
| AZ91_1DOC | TID (1.0 mm DOC) | 166 ± 5 |
| AZ91_1.5DOC | TID (1.5 mm DOC) | 185 ± 3 |
| ZK60_AR | Conventional | 136 ± 7 |
| ZK60_HS | TID (high cutting speed) | 141 ± 6 |
| ZK60_MS | TID (medium cutting speed) | 151 ± 9 |
| ZK60_LS | TID (low cutting speed) | 157 ± 2 |
| Material | Fabrication Method | Average Grain Size (µm) |
|---|---|---|
| AZ91_AR | Conventional | 3.17 ± 0.86 |
| AZ91_0.5DOC | TID (0.5 mm DOC) | 1.82 ± 0.44 |
| AZ91_1DOC | TID (1.0 mm DOC) | 1.54 ± 0.43 |
| AZ91_1.5DOC | TID (1.5 mm DOC) | 1.27 ± 0.34 |
| Mg/1Fe3O4 DMD | Conventional | 16 ± 6 |
| Mg/2Fe3O4 DMD | Conventional | 8 ± 3 |
| Mg/3Fe3O4 DMD | Conventional | 10 ± 4 |
| Mg/1Fe3O4 TID | TID (one set of parameters) | 8 ± 2 |
| Mg/2Fe3O4 TID | TID (one set of parameters) | 8 ± 3 |
| Mg/3Fe3O4 TID | TID (one set of parameters) | 9 ± 3 |
| ZK60_AR | Conventional | 0.99 ± 0.47 |
| ZK60_HS | TID (high cutting speed) | 1.13 ± 0.45 |
| ZK60_MS | TID (medium cutting speed) | 1.05 ± 0.29 |
| ZK60_LS | TID (low cutting speed) | 0.89 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johanes, M.; Gupta, M. The Promise of Turning Induced Deformation Process for Synthesizing Magnesium Based Materials with Superior Mechanical Response. Technologies 2021, 9, 69. https://doi.org/10.3390/technologies9040069
Johanes M, Gupta M. The Promise of Turning Induced Deformation Process for Synthesizing Magnesium Based Materials with Superior Mechanical Response. Technologies. 2021; 9(4):69. https://doi.org/10.3390/technologies9040069
Chicago/Turabian StyleJohanes, Michael, and Manoj Gupta. 2021. "The Promise of Turning Induced Deformation Process for Synthesizing Magnesium Based Materials with Superior Mechanical Response" Technologies 9, no. 4: 69. https://doi.org/10.3390/technologies9040069
APA StyleJohanes, M., & Gupta, M. (2021). The Promise of Turning Induced Deformation Process for Synthesizing Magnesium Based Materials with Superior Mechanical Response. Technologies, 9(4), 69. https://doi.org/10.3390/technologies9040069