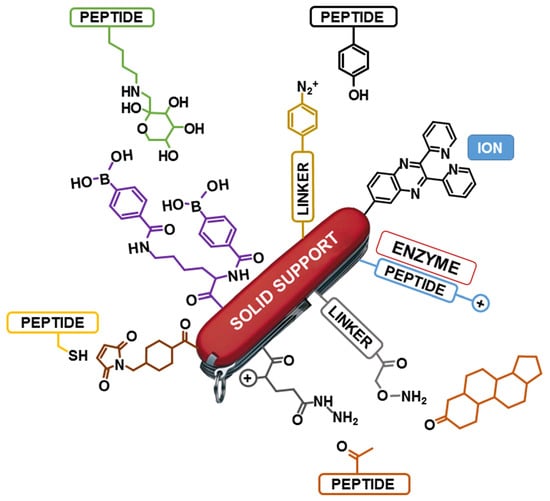
Veni, Vidi, Vici: Immobilized Peptide-Based Conjugates as Tools for Capture, Analysis, and Transformation
Abstract
:1. Introduction
2. Immobilized Peptides as Multifunctional Tools
2.1. Capturing Cysteine-Containing Peptides
2.2. Capturing Carbonylated Compounds
2.3. Boronate Affinity Separation for Glycoconjugates Capture
2.4. Capturing Tyrosine-Containing Peptides by Diazonium Salts
2.5. Enzyme Specificity and Activity Analysis
2.6. Immobilized Heterocyclic Peptide Conjugates as Ligands
3. Discussion: Peptide Biosensors—Advantages and Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, N.L.; Anderson, N.G. Proteome and proteomics: New technologies, new concepts, and new words. Electrophoresis 1998, 19, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Pedrero-Prieto, C.M.; García-Carpintero, S.; Frontiñán-Rubio, J.; Llanos-González, E.; Aguilera García, C.; Alcaín, F.J.; Lindberg, I.; Durán-Prado, M.; Peinado, J.R.; Rabanal-Ruiz, Y. A comprehensive systematic review of CSF proteins and peptides that define Alzheimer’s disease. Clin. Proteom. 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, S.; Chen, Z.; Adhikari, B.K.; Zhang, Y.; Zhang, J.; Sun, J.; Wang, Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin. Chim. Acta 2020, 510, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, J.; Slebos, R.J.C.; Liebler, D.C. Cysteinyl peptide capture for shotgun proteomics: Global assessment of chemoselective fractionation. J. Proteome Res. 2010, 9, 5461–5472. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, T.E.; Jensen, O.N.; Robinson, P.J.; Larsen, M.R. SIMAC (sequential elution from IMAC), a phosphoproteomics strategy for the rapid separation of monophosphorylated from multiply phosphorylated peptides. Mol. Cell. Proteom. MCP 2008, 7, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Tutturen, A.E.; Holm, A.; Jørgensen, M.; Stadtmüller, P.; Rise, F.; Fleckenstein, B. A technique for the specific enrichment of citrulline-containing peptides. Anal. Biochem. 2010, 403, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Kijewska, M.; Nuti, F.; Wierzbicka, M.; Waliczek, M.; Ledwoń, P.; Staśkiewicz, A.; Real-Fernandez, F.; Sabatino, G.; Rovero, P.; Stefanowicz, P.; et al. An Optimised Di-Boronate-ChemMatrix Affinity Chromatography to Trap Deoxyfructosylated Peptides as Biomarkers of Glycation. Molecules 2020, 25, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, M.; Kuczer, M. New Hydrazine Derivatives as Derivatization Reagents in The Analysis of Carbonyl Compounds Using MS. In Proceedings of the XIV Copernicus Doctoral Seminar, Toruń, Poland, 20–22 September 2021; Volume 71. [Google Scholar]
- Bąchor, R.; Mielczarek, P.; Rudowska, M.; Silberring, J.; Szewczuk, Z. Sensitive detection of charge derivatized peptides at the attomole level using nano-LC-ESI-MRM analysis. Int. J. Mass Spectrom. 2014, 362, 32–38. [Google Scholar] [CrossRef]
- Benito, J.M.; Christensen, C.A.; Meldal, M. Versatile solid-phase synthesis of peptide-derived 2-oxazolines. Application in the synthesis of ligands for asymmetric catalysis. Org. Lett. 2005, 7, 581–584. [Google Scholar] [CrossRef]
- Burns, A.; Olszowy, P.; Ciborowski, P. Chapter 2: Biomolecules. In Proteomic Profiling and Analytical Chemistry; Ciborowski, P., Silberring, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 7–24. [Google Scholar]
- Bak, D.W.; Bechtel, T.J.; Falco, J.A.; Weerapana, E. Cysteine reactivity across the subcellular universe. Curr. Opin. Chem. Biol. 2019, 48, 96–105. [Google Scholar] [CrossRef]
- Liu, T.; Qian, W.J.; Strittmatter, E.F.; Camp, D.G.; Anderson, G.A.; Thrall, B.D.; Smith, R.D. High-throughput comparative proteome analysis using a quantitative cysteinyl-peptide enrichment technology. Anal. Chem. 2004, 76, 5345–5353. [Google Scholar] [CrossRef]
- Baldwin, A.D.; Kiick, K.L. Tunable degradation of maleimide-thiol adducts in reducing environments. Bioconjug. Chem. 2011, 22, 1946–1953. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, G.; Lecolley, F.; Tao, L.; Haddleton, D.M.; Clerx, J.; Cornelissen, J.J.; Velonia, K. Design and synthesis of N-maleimido-functionalized hydrophilic polymers via copper-mediated living radical polymerization: A suitable alternative to PEGylation chemistry. J. Am. Chem. Soc. 2005, 127, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Gevrek, T.N.; Sanyal, R.; Sanyal, A. Indispensable platforms for bioimmobilization: Maleimide-based thiol reactive hydrogels. Bioconjug. Chem. 2014, 25, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Gygi, S.P.; Rist, B.; Gerber, S.A.; Turecek, F.; Gelb, M.B.; Aebersold, R. Quantitative analysis of complex protein mixtures using isotope-coded affnity tags. Nat. Biotechnol. 1999, 17, 994–999. [Google Scholar] [CrossRef]
- Ren, D.; Julka, S.; Inerowicz, H.D.; Regnier, F.E. Enrichment of cysteine-containing peptides from tryptic digests using a quaternary amine tag. Anal. Chem. 2004, 76, 4522–4530. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, R.; Gorzeń, O.; Rola, A.; Mojsa, K.; Panek-Laszczyńska, K.; Konieczny, A.; Dąbrowska, K.; Witkiewicz, W.; Szewczuk, Z. Enrichment of Cysteine-Containing Peptide by On-Resin Capturing and Fixed Charge Tag Derivatization for Sensitive ESI-MS Detection. Molecules 2020, 25, 1372. [Google Scholar] [CrossRef] [Green Version]
- Waliczek, M.; Kijewska, M.; Rudowska, M.; Setner, B.; Stefanowicz, P.; Szewczuk, Z. Peptides labeled with pyridinium salts for sensitive detection and sequencing by electrospray tandem mass spectrometry. Sci. Rep. 2016, 6, 37720. [Google Scholar] [CrossRef]
- Kadir, A.A.; Clarke, K.; Evans, R.D. Cardiac ketone body metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165739. [Google Scholar] [CrossRef] [PubMed]
- Shigeri, Y.; Yasuda, A.; Sakai, M.; Ikeda, S.; Arakawa, R.; Sato, H.; Kinumi, T. Hydrazide and hydrazine reagents as reactive matrices for matrix-assisted laser desorption/ionization mass spectrometry to detect steroids with carbonyl groups. Eur. J. Mass Spectrom. 2015, 21, 79–90. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Barber, D.S.; Gavin, T. Molecular Mechanisms of the Conjugated α,β-Unsaturated Carbonyl Derivatives: Relevance to Neurotoxicity and Neurodegenerative Diseases. Toxicol. Sci. 2008, 104, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kurpet, K.; Chwatko, G. New Psychoactive Substances—Cathinone and Its Derivatives. Wiadomości Chem. 2019, 73, 461–489. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Mirzaei, H.; Baena, B.; Barbas, C.; Regnier, F. Identification of oxidized proteins in rat plasma using avidin chromatography and tandem mass spectrometry. Proteomics 2008, 8, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Regnier, F. Enrichment of Carbonylated Peptides Using Girard P Reagent and Strong Cation Exchange Chromatography. Anal. Chem. 2006, 78, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Hoffmann, R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal. Bioanal. Chem. 2010, 397, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Kijewska, M.; Koch, T.; Waliczek, M.; Konieczny, A.; Stefanowicz, P.; Szewczuk, Z. Selective ESI-MS detection of carbonyl containing compounds by aminooxyacetic acid immobilized on a resin. Anal. Chim. Acta 2021, 1176, 338767. [Google Scholar] [CrossRef] [PubMed]
- Setner, B.; Szewczuk, Z. New ionization tags based on the structure of the 5-azoniaspiro[4.4]nonyl tag for a sensitive peptide sequencing by mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Setner, B.; Stefanowicz, P.; Szewczuk, Z. Quaternary ammonium isobaric tag for a relative and absolute quantification of peptides. J. Mass Spectrom. 2018, 53, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, P.; Kluczyk, A.; Szewczuk, Z. Derivatization of peptides for improved detection by mass spectrometry. Amino Acids Pept. Proteins 2016, 40, 36–74. [Google Scholar]
- Setner, B.; Rudowska, M.; Klem, E.; Cebrat, M.; Szewczuk, Z. Peptides derivatized with bicyclic quaternary ammonium ionization tags. Sequencing via tandem mass spectrometry. J. Mass Spectrom. 2014, 49, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Cydzik, M.; Rudowska, M.; Stefanowicz, P.; Szewczuk, Z. Derivatization of peptides as quaternary ammonium salts for sensitive detection by ESI-MS. J. Pept. Sci. 2011, 17, 445–453. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, P.; Cerami, A. Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 2001, 56, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Girones, X.; Guimera, A.; Cruz-Sanchez, C.Z.; Ortega, A.; Sasaki, N.; Makita, Z.; Lafuente, J.V.; Kalaria, R.; Cruz-Sanchez, F.F. Nε-Carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer’s disease. Free Radic. Biol. Med. 2004, 36, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef]
- Alt, N.; Carson, J.A.; Alderson, N.L.; Wang, Y.; Nagai, R.; Henle, T. Chemical modification of muscle protein in diabetes. Arch. Biochem. Biophys. 2004, 425, 200–206. [Google Scholar] [CrossRef]
- Soboleva, A.; Mavropulo-Stolyarenko, G.; Karonova, T.; Thieme, D.; Hoehenwarter, W.; Ihling, C.; Stefanov, V.; Grishina, T.; Frolov, A. Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 2329. [Google Scholar] [CrossRef] [Green Version]
- Madian, G.A.; Regnier, F.E. Protein identyfiaction of carbonylated proteins and their oxidation sites. J. Proteome Res. 2010, 9, 3766–3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soboleva, A.; Modzel, M.; Didio, A.; Płóciennik, H.; Kijewska, M.; Grischina, T.; Karonova, T.; Bilova, T.; Stefanov, V.; Stefanowicz, P.; et al. Quantification of prospective type 2 diabetes mellitus biomarkers by stable isotope dilution with bi-labeled standard glycated peptides. Anal. Methods 2017, 9, 409–418. [Google Scholar] [CrossRef]
- Madera, M.; Mechref, Y.; Klouckova, I.; Novotny, M.V. Semiautomated high-sensitivity profiling of human blood serum glycoproteins through lectin preconcentration and multidimensional chromatography/tandem mass spectrometry. J. Proteome Res. 2006, 5, 2348–2363. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, X.N.; Sun, D.G.; Han, G.H.; Wang, F.J.; Ye, M.L.; Wang, L.M.; Zou, H.F. Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J. Proteome Res. 2009, 8, 651–661. [Google Scholar] [CrossRef]
- Wada, Y.; Tajiri, M.; Yoshida, S. Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal. Chem. 2004, 76, 6560–6565. [Google Scholar] [CrossRef]
- Liu, Z.; He, H. Synthesis and Applications of Boronate Affinity Materials: From Class Selectivity to Biomimetic Specificity. Acc. Chem. Res. 2017, 50, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; He, H.; Liu, Z. Recent progress and application of boronate affinity materials in bioanalysis. Trends Anal. Chem. 2021, 140, 116271. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, G.W.; Jain, R.; Pippin, J.W.; Shankland, S.J.; Pun, S.H. Boronic acid copolymers for direct loading and acid-triggered release of Bis-T-23 in cultured podocytes. ACS Biomater.-Sci. Eng. 2018, 4, 3968–3973. [Google Scholar] [CrossRef]
- Gao, L.; Du, J.; Wang, C.Z.; Wei, Y. Fabrication of a dendrimer-modified boronate affinity material for online selective enrichment of cis-diol-containing compounds and its application in determination of nucleosides in urine. RSC Adv. 2015, 5, 106161–106170. [Google Scholar] [CrossRef]
- Frolov, A.; Blüher, M.; Hoffmann, R. Glycation sites of human plasma proteins are affected to different extents by hyperglycemic conditions in type 2 diabetes mellitus. Anal. Bioanal. Chem. 2014, 406, 5755–5763. [Google Scholar] [CrossRef]
- Kijewska, M.; Kuc, A.; Kluczyk, A.; Waliczek, M.; Man-Kupisinska, A.; Łukasiewicz, J.; Stefanowicz, P.; Szewczuk, Z. Selective detection of carbohydrates and their peptide conjugates by ESI-MS using synthetic quaternary ammonium salt derivatives of phenylboronic acids. J. Am. Soc. Mass Spectrom. 2014, 25, 966–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.; Liu, Z.; Liu, Y.; Dou, P.; Chen, H. Ring-opening polymerization with synergistic co-monomers: Access to a boronate-functionalized polymeric monolith for the specific capture of cis-diol-containing biomolecules under neutral conditions. Angew. Chem. Int. Ed. 2009, 48, 6704–6707. [Google Scholar] [CrossRef]
- Li, X.; Pennington, J.; Stobaugh, J.F.; Schöneich, C. Synthesis of Sulfonamide- and Sulfonyl-Phenylboronic Acid-Modified Silica Phases for Boronate Affinity Chromatography at Physiological pH. Anal. Biochem. 2008, 372, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berube, M.; Dowlut, M.; Hall, D.G. Benzoboroxoles as Efficient Glycopyranoside-Binding Agents in Physiological Conditions: Structure and Selectivity of Complex Formation. J. Org. Chem. 2008, 73, 6471–6479. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Wang, S.; Ye, J.; Nie, H.; Liu, Z. Pyridinylboronic Acid-Functionalized Organic-Silica Hybrid Monolithic Capillary for the Selective Enrichment and Separation of cis-Diol-Containing Biomolecules at Acidic pH. J. Chromatogr. A 2014, 1339, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, Z.; Dong, M.; Ye, M.; Zou, H. Synthesis and characterization of a new boronate affinity monolithic capillary for specific capture of cis-diol-containing compounds. J. Chromatogr. A 2009, 1216, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.; Ha, M.Y.; Lee, E.K.; Choo, J. Immobilization of aminophenylboronic acid on magnetic beads for the direct determination of glycoproteins by matrix assisted laser desorption ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2005, 16, 1456–1460. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wu, Z.; Zhang, L.; Lu, H.; Yang, P.; Webley, P.A.; Zhao, D. Highly specific enrichment of glycopeptides using boronic acid-functionalized mesoporous silica. Anal. Chem. 2009, 81, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhang, H.; Tang, J.; Deng, C.; Zhang, X. Facile synthesis of mercaptophenylboronic acid-functionalized core-shell structure Fe3O4@C@Au magnetic microspheres for selective enrichment of glycopeptides and glycoproteins. J. Phys. Chem. C 2010, 114, 9221–9226. [Google Scholar] [CrossRef]
- Wang, H.; Bie, Z.; Lu, C.; Liu, Z. Magnetic nanoparticles with dendrimer-assisted boronate avidity for the selective enrichment of trace glycoproteins. Chem. Sci. 2013, 4, 4298–4303. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, B.; Weng, Y.; Liu, J.; Li, S.; Hu, Y.; Yang, K.; Liang, Z.; Zhang, L.; Zhang, Y. 3-Carboxybenzoboroxole functionalized polyethylenimine modified magnetic graphene oxide nanocomposites for human plasma glycoproteins enrichment under physiological conditions. Anal. Chem. 2018, 90, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, W.; Smeekens, J.M.; Wu, R. An enrichment method based on synergistic and reversible covalent interactions for large-scale analysis of glycoproteins. Nat. Commun. 2018, 9, 1692–1703. [Google Scholar] [CrossRef] [Green Version]
- Bie, Z.; Chen, Y.; Li, H.; Wu, R.; Liu, Z. Off-line hyphenation of boronate affinity monolith-based extraction with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for efficient analysis of glycoproteins/glycopeptides. Anal. Chim. Acta 2014, 834, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, D.; Sun, L.; Yao, Y.; Yao, C. A designable aminophenylboronic acid functionalized magnetic Fe3O4/ZIF-8/APBA for specific recognition of glycoproteins and glycopeptides. RSC Adv. 2018, 8, 6887–6892. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.; Chen, Q.; He, J.; Li, Z.; Yu, L.; Lan, F.; Wu, Y. Boronic acid-functionalized magnetic metal-organic frameworks via a dual-ligand strategy for highly efficient enrichment of phosphopeptides and glycopeptides. ACS Sustain. Chem. Eng. 2019, 7, 6043–6052. [Google Scholar] [CrossRef]
- Uziel, M.; Smith, L.H.; Taylor, S.A. Modified nucleosides in urine: Selective removal and analysis. Clin. Chem. 1976, 22, 1451–1455. [Google Scholar] [CrossRef]
- Howard, A.N.; Wild, F. The reactions of diazonium compounds with amino acids and proteins. Biochem. J. 1957, 65, 651–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, E. Synthesis of azobenzenes: The coloured pieces of molecular materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J. Rational Selection of Oral 5-Aminosalicylate Formulations and Prodrugs for the Treatment of Ulcerative Colitis. Am. J. Gastroenterol. 2002, 97, 2939. [Google Scholar] [CrossRef]
- Yousaf, A.; Abd Shafida, H.; Umer, R. Biomedical Applications of Aromatic Azo Compounds. Mini-Rev. Med. Chem. 2018, 18, 1548–1558. [Google Scholar]
- Kaur, H.; Yadav, S.; Narasimhan, B. Diazenyl derivatives and their complexes as anticancer agents. Anti-Cancer Agent. Med. Chem. 2018, 16, 1240–1265. [Google Scholar] [CrossRef]
- Mutlu, H.; Geiselhart, C.M.; Barner-Kowollik, C. Untapped potential for debonding on demand: The wonderful world of azo-compounds. Mater. Horiz. 2018, 5, 162–183. [Google Scholar] [CrossRef]
- Siemion, I.Z.; Szewczuk, Z.; Herman, Z.S.; Stachura, Z. To the problem of biologically active conformation of enkephalin. Mol. Cell. Biochem. 1981, 34, 23–29. [Google Scholar] [CrossRef]
- Saffran, M.; Kumar, G.S.; Savariar, C.; Burnham, J.C.; Williams, F.; Neckers, D.C. A new approach to the oral administration of insulin and other peptides. Science 1986, 233, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Trader, D.J.; Carlson, E.E. Chemoselective hydroxyl group transformation: An elusive target. Mol. BioSyst. 2012, 8, 2484–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, S.; Chandrasekaran, S. Modification of amino acids using arenediazonium salts. Org. Biomol. Chem. 2019, 17, 8308–8329. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Nie, Y.; Ye, F.; Zhang, M.; Xia, J. Site Selective Azo Coupling for Peptide Cyclization and Affinity Labeling of an SH3 Protein. Bioconjug. Chem. 2015, 26, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Wang, Y.; Yu, Z.; Cong, Q.; Han, X.X.; Zhao, B. A rapid and ultrasensitive SERRS assay for histidine and tyrosine based on azo coupling. Talanta 2016, 159, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Popiel, D.; Kuczer, M.; Biernat, M. Azopeptides—New synthetic challenge. In Proceedings of the XIV Copernicus Doctoral Seminar, Toruń, Poland, 20–22 September 2021; Volume 28. [Google Scholar]
- Fridkin, G.; Rahimipour, S.; Ben-Aroya, N.; Kapitkovsky, A.; Di-Segni, S.; Rosenberg, M.; Kustanovich, I.; Koch, Y.; Gilon, C.; Fridkin, M. Novel cyclic azo-bridged analogs of gonadotropin-releasing hormone. J. Pept. Sci. 2006, 12, 106–115. [Google Scholar] [CrossRef]
- Roldo, M.; Barbu, E.; Brown, J.F.; Laight, D.W.; Smart, J.D.; Tsibouklis, J. Azo compounds in colon-specific drug delivery. Expert Opin. Drug Deliv. 2007, 4, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Poręba, M.; Drąg, M. Current strategies for probing substrate specificity of proteases. Curr. Med. Chem. 2010, 17, 3968–3995. [Google Scholar] [CrossRef]
- Deu, E.; Verdoes, M.; Bogyo, M. New approaches for dissecting protease functions to improve probe development and drug discovery. Nat. Struct. Mol. Biol. 2012, 19, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.L.; Backes, B.J.; Leonetti, F.; Mahrus, S.; Ellman, J.A.; Craik, C.S. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc. Natl. Acad. Sci. USA 2000, 97, 7754–7759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drag, M.; Bogyo, M.; Ellman, J.A.; Salvesen, G.S. Aminopeptidase fingerprints, an integrated approach for identification of good substrates and optimal inhibitors. J. Biol. Chem. 2010, 285, 3310–3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poreba, M.; McGowan, S.; Skinner-Adams, T.S.; Trenholme, K.R.; Gardiner, D.L.; Whisstock, J.C.; To, J.; Salvesen, G.S.; Dalton, J.P.; Drag, M. Fingerprinting the substrate specificity of m1 and m17 aminopeptidases of human malaria, plasmodium falciparum. PLoS ONE 2012, 7, e31938. [Google Scholar] [CrossRef] [Green Version]
- Kasperkiewicz, P.; Poreba, M.; Snipas, S.J.; Parker, H.; Winterbourn, C.C.; Salvesen, G.S.; Drag, M. Design of ultrasensitive probes for human neutrophil elastase through hybrid combinatorial substrate library profiling. Proc. Natl. Acad. Sci. USA 2014, 18, 2518–2523. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, T.; Ono, H.; Kikuchi, K.; Nirasawa, S.; Takahashi, S. Purification and characterization of aspartic protease derived from Sf9 insect cells. Biosci. Biotechnol. Biochem. 2010, 74, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, M.; Gruba, N.; Miecznikowska, A.; Popow-Stelmaszyk, J.; Gütschow, M.; Stirnberg, M.; Furtmann, N.; Bajorath, J.; Lesner, A.; Rolka, K. Substrate specificity of human matriptase-2. Biochimie 2014, 97, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Silva, V.O.; Okamoto, D.N.; Kondo, M.Y.; Santos, S.M.; Hirata, I.Y.; Vallim, M.A.; Pascon, R.C.; Gouvea, I.E.; Juliano, M.A.; et al. Internally quenched fluorescent peptide libraries with randomized sequences designed to detect endopeptidases. Anal. Biochem. 2012, 421, 299–307. [Google Scholar] [CrossRef]
- Stocker, W.; Ng, M.; Auld, D.S. Fluorescent oligopeptide substrates for kinetic characterization of the specificity of Astacus protease. Biochemistry 1990, 29, 10418–10425. [Google Scholar] [CrossRef]
- Lutzner, N.; Kalbacher, H. Quantifying cathepsin S activity in antigen presenting cells using a novel specific substrate. J. Biol. Chem. 2008, 283, 36185–36194. [Google Scholar] [CrossRef] [Green Version]
- Poręba, M.; Szalek, A.; Rut, W.; Kasperkiewicz, P.; Rutkowska-Wlodarczyk, I.; Snipas, S.J.; Itoh, Y.; Turk, D.; Turk, B.; Overall, C.M.; et al. Highly sensitive and adaptable fluorescence-quenched pair discloses the substrate specificity profiles in diverse protease families. Sci. Rep. 2017, 7, 43135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.W.; Yang, L.M.; Wang, Q.Q. Lanthanide-Coded Protease-Specific Peptide–Nanoparticle Probes for a Label-Free Multiplex Protease Assay Using Element Mass Spectrometry: A Proof-of-Concept Study. Angew. Chem. Int. Ed. 2011, 50, 5130–5133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabelski, M.; Rogiewicz, M.; Wiczk, W. Fluorogenic peptide substrates containing benzoxazol-5-yl-alanine derivatives for kinetic assay of cysteine proteases. Anal. Biochem. 2005, 342, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ellard, J.M.; Zollitsch, T.; Cummins, W.J.; Hamilton, A.L.; Bradley, M. Fluorescence enhancement through enzymatic cleavage of internally quenched dendritic peptides: A sensitive assay for the AspN endoproteinase. Angew. Chem. Int. Ed. 2002, 41, 3233–3236. [Google Scholar] [CrossRef]
- Akers, W.J.; Xu, B.G.; Lee, H.; Sudlow, G.P.; Fields, G.B.; Achilefu, S.; Edwards, W.B. Detection of MMP-2 and MMP-9 Activity in Vivo with a Triple-Helical Peptide Optical Probe. Bioconjug. Chem. 2012, 23, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippova, I.Y.; Lysogorskaya, E.N.; Anisimova, V.V.; Suvorov, L.I.; Oksenoit, E.S.; Stepanov, V.M. Fluorogenic peptide substrates for assay of aspartyl proteinases. Anal. Biochem. 1996, 234, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.T.; Chung, C.C.; Holzman, T.F.; Krafft, G.A. A continuous fluorescence assay of renin activity. Anal. Biochem. 1993, 210, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Thornberry, N.A. Synthesis of a fluorogenic interleukin-1 beta converting enzyme substrate based on resonance energy transfer. Pept. Res. 1994, 7, 72–76. [Google Scholar] [PubMed]
- Holskin, B.P.; Bukhtiyarova, M.; Dunn, B.M.; Baur, P.; Dechastonay, J.; Pennington, M.W. A continuous fluorescence-based assay of human cytomegalovirus protease using a peptide substrate. Anal. Biochem. 1995, 227, 148–155. [Google Scholar] [CrossRef]
- Chen, P.T.; Liao, T.Y.; Hu, C.J.; Wu, S.T.; Wang, S.S.-S.; Chen, R.P.-Y. A highly sensitive peptide substrate for detecting two Aβ-degrading enzymes: Neprilysin and insulin-degrading enzyme. J. Neurosci. Methods 2010, 190, 57–62. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Bergstedt, T.; Shi, X.B.; Rininsland, F.; Kushon, S.; Xia, W.S.; Ley, K.; Achyuthan, K.; McBranch, D.; Whitten, D. Fluorescent-conjugated polymer superquenching facilitates highly sensitive detection of proteases. Proc. Natl. Acad. Sci. USA 2004, 101, 7511–7515. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Geng, J.; Ren, J.; Miyoshi, D.; Sugimoto, N.; Qu, X. Label-free colorimetric and quantitative detection of cancer marker protein using non-crosslinking aggregation of Au/Ag nanoparticles induced by target-specific peptide probe. Biosens. Bioelectron. 2011, 26, 4804–4809. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, H.S.; Choi, J.W.; Hong, J.W.; Kim, Y.K.; Oh, B.K. A Novel Au-Nanoparticle Biosensor for the Rapid and Simple Detection of PSA Using a Sequence-Specific Peptide Cleavage Reaction. Biosens. Bioelectron. 2013, 49, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halling, P.J. Understanding enzyme action at solid surfaces. Biocatalysis 2006, 34, 309–311. [Google Scholar]
- Babiak, P.; Reymond, J.-L. A High-Throughput, Low-Volume Enzyme Assay on Solid Support. Anal. Chem. 2005, 77, 373–377. [Google Scholar] [CrossRef]
- Milićević, D.; Hlaváč, J. Immobilized fluorescent probes for simultaneous multiple protease detection. Chemosensors 2021, 9, 119. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, K.L. Oligopeptide immobilization strategy for improving stability and sensitivity of liquid-crystal protease assays. Sens. Actuators B Chem. 2014, 204, 734–740. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhang, Q.; Chu, X.; Chen, T.T.; Ge, J.; Yu, R.Q. Graphene oxide–peptide conjugate as an intracellular protease sensor for caspase-3 activation imaging in live cells. Angew. Chem. Int. Ed. 2011, 50, 7065–7069. [Google Scholar] [CrossRef]
- Kapprell, H.P.; Maurer, A.; Kramer, F.; Heinrich, B.; Buenning, C.; Narvaez, A.; Kalbacher, H.; Flad, T. Development of a fluorescence resonance energy transfer peptide library technology for detection of protease contaminants in protein-based raw materials used in diagnostic assays. Assay Drug Dev. Technol. 2011, 9, 549–553. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Liu, Z.Y.; Hu, L.Z.; Yuan, Y.L.; Xu, G.B. Turn-on fluorescence sensor based on single-walled-carbon-nanohorn-peptide complex for the detection of thrombin. Chem.-Eur. J. 2012, 18, 16556–16561. [Google Scholar] [CrossRef] [PubMed]
- Kaman, W.E.; Hulst, A.G.; van Alphen, P.T.W.; Roffel, S.; van der Schans, M.J.; Merkel, T.; van Belkum, A.; Bikker, F.J. Peptide-based fluorescence resonance energy transfer protease substrates for the detection and diagnosis of bacillus species. Anal. Chem. 2011, 83, 2511–2517. [Google Scholar] [CrossRef]
- Fudala, R.; Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K.; Gryczynski, Z.; Borejdo, J.; Sarkar, P.; Gryczynski, I. Fluorescence detection of MMP-9. I. MMP-9 selectively cleaves Lys-Gly-Pro-Arg-Ser-Leu-Ser-Gly-Lys peptide. Curr. Pharm. Biotechnol. 2011, 12, 834–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, J.; Chung, L.P.; Ariese, F.; Irth, H.; Gooijer, C. Coupling of size-exclusion chromatography to a continuous assay for Subtilisin using a fluorescence resonance energy transfer peptide substrate: Testing of two standard inhibitors. J. Chromatogr. A 2005, 1081, 140–144. [Google Scholar] [CrossRef]
- Pham, W.; Choi, Y.D.; Weissleder, R.; Tung, C.H. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjug. Chem. 2004, 15, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.H.; Gerszten, R.E.; Jaffer, F.A.; Weissleder, R. A novel near-infrared fluorescence sensor for detection of thrombin activation in blood. ChemBioChem 2002, 3, 207–211. [Google Scholar] [CrossRef]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; He, Y.Q.; Mao, X.; Sun, Y.H.; Zhang, X.B.; Li, C.Z.; Lin, Y.H.; Liu, G.D. Electrochemical assay of active prostate-specific antigen (PSA) using ferrocene-functionalized peptide probes. Electrochem. Commun. 2010, 12, 471–474. [Google Scholar] [CrossRef]
- Adjemian, J.; Anne, A.; Cauet, G.; Demaille, C. Cleavage-sensing redox peptide monolayers for the rapid measurement of the proteolytic activity of trypsin and α-thrombin enzymes. Langmuir 2010, 26, 10347–10356. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, Y.L.; Yan, F.; Ding, L.; Ding, S.J.; Ju, H.X.; Yin, Y.B. Ultrasensitive scanometric strategy for detection of matrix metalloproteinases using a histidine tagged peptide–Au nanoparticle probe. Chem. Commun. 2011, 47, 2877–2879. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.L.; Wang, C.; Qiu, X.Y.; Gao, Q.; Zhang, C.X. Reagent-less electrogenerated chemiluminescence peptide-based biosensor for the determination of prostate-specific antigen. Talanta 2012, 100, 162–167. [Google Scholar] [CrossRef]
- Qi, H.L.; Li, M.; Dong, M.M.; Ruan, S.P.; Gao, Q.; Zhang, C.X. Electrogenerated chemiluminescence peptide-based biosensor for the determination of prostate-specific antigen based on target-induced cleavage of peptide. Anal. Chem. 2014, 86, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, R.; Cydzik, M.; Rudowska, M.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Sensitive electrospray mass spectrometry analysis of one-bead-one-compound peptide libraries labeled by quaternary ammonium salts. Mol. Divers. 2012, 16, 613–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bąchor, R.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. New method of peptide cleavage based on Edman degradation. Mol. Divers. 2013, 17, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, G. Combinatorial Chemistry. Chem. Soc. Rev. 1995, 24, 309–317. [Google Scholar] [CrossRef]
- Furka, A.; Sebestyen, F.; Asgedom, M.; Dibo, G. General method for rapid synthesis of multicomponent peptide mixtures. Int. J. Pept. Res. 1991, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, R.; Waliczek, M.; Stefanowicz, P.; Szewczuk, Z. Trends in the design of new isobaric labeling reagents for quantitative proteomics. Molecules 2019, 24, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hintersteiner, M.; Buehler, C.; Auer, M. On-bead screens sample narrower affinity ranges of protein–ligand interactions compared to equivalent solution assays. ChemPhysChem 2012, 13, 3472–3480. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Casini, A. Design Strategies and Medicinal Applications of Metal-Peptidic Bioconjugates. Bioconjug. Chem. 2020, 31, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Kadej, A.; Kuczer, M.; Czarniewska, E.; Urbański, A.; Rosiński, G.; Kowalik-Jankowska, T. High stability and biological activity of the copper(II) complexes of alloferon 1 analogues containing tryptophan. J. Inorg. Biochem. 2016, 163, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Kotynia, A.; Cebrat, M.; Brasuń, J. The Analysis of the Structural Aspects of Cu(II) Binding by Cyclic His/Asp-Analogues of Somatostatin. Int. J. Pept. Res. Ther. 2020, 26, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.Y.; Adamek, R.N.; Dick, B.L.; Credille, C.V.; Morrison, C.N.; Cohen, S.M. Targeting Metalloenzymes for Therapeutic Intervention. Chem. Rev. 2019, 119, 1323–1455. [Google Scholar] [CrossRef] [PubMed]
- Padjasek, M.; Kocyła, A.; Kluska, K.; Kerber, O.; Tran, J.B.; Krężel, A. Structural zinc binding sites shaped for greater works: Structure-function relations in classical zinc finger, hook and clasp domains. J. Inorg. Biochem. 2020, 204, 110955. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, G.; König, B. The Use of Solid-Phase Synthesis Techniques for the Preparation of Peptide–Metal Complex Conjugates. Eur. J. Org. Chem. 2008, 2008, 597–634. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Koprowska-Ratajska, M.; Kluczyk, A.; Stefanowicz, P.; Bartosz-Bechowski, H.; Szewczuk, Z. Solid phase synthesis of peptides containing novel amino acids, substituted 3-benzimidazolealanines. Amino Acids 2009, 36, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Staszewska, A.; Stefanowicz, P.; Szewczuk, Z. Direct solid-phase synthesis of quinoxaline-containing peptides. Tetrahedron Lett. 2005, 46, 5525–5528. [Google Scholar] [CrossRef]
- Reetz, M.T. Combinatorial Transition-Metal Catalysis: Mixing Monodentate Ligands to Control Enantio-, Diastereo-, and Regioselectivity. Angew. Chem. Int. Ed. 2008, 47, 2556–2588. [Google Scholar] [CrossRef] [PubMed]
- Křupková, S.; Funk, P.; Soural, M.; Hlaváč, J. 4-Chloro-2-Fluoro-5-Nitrobenzoic Acid as a Possible Building Block for Solid-phase Synthesis of Various Heterocyclic Scaffolds. ACS Comb. Sci. 2013, 15, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.A.; Meldal, M. Solid-phase synthesis of a peptide-based P,S-ligand system designed for generation of combinatorial catalyst libraries. J. Comb. Chem. 2007, 9, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, M.; Hureau, C.; Gras, E.; Faller, P. Coordination complexes and biomolecules: A wise wedding for catalysis upgrade. Coord. Chem. Rev. 2016, 308, 445–459. [Google Scholar] [CrossRef]
- Sista, P.; Ghosh, K.; Martinez, J.S.; Rocha, R.C. Metallo-Biopolymers: Conjugation Strategies and Applications. Polym. Rev. 2014, 54, 627–676. [Google Scholar] [CrossRef]
- Albada, B.; Metzler-Nolte, N. Organometallic–Peptide Bioconjugates: Synthetic Strategies and Medicinal Applications. Chem. Rev. 2016, 116, 11797–11839. [Google Scholar] [CrossRef] [PubMed]
- Kluczyk, A.; Staszewska, A.; Stefanowicz, P.; Bartosz-Bechowski, H.; Koprowska, M.; Szewczuk, Z. Novel heterocyclic amino acids: Post-assembly on-resin modifications of peptides. In Proceedings of the 29th European Peptide Symposium, Gdansk, Poland, 3–8 September 2006; pp. 346–347. [Google Scholar]
- Aldridge, W.S.; Hornstein, B.J.; Serron, S.; Dattelbaum, D.M.; Schoonover, J.R.; Meyer, T.J. Synthesis and characterization of oligoproline-based molecular assemblies for light harvesting. J. Org. Chem. 2006, 71, 5186–5190. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.M.; Coggins, M.K.; Concepcion, J.J.; Ashford, D.L.; Fang, Z.; Alibabaei, L.; Ma, D.; Meyer, T.J.; Waters, M.L. Synthesis and electrocatalytic water oxidation by electrode-bound helical peptide chromophore-catalyst assemblies. Inorg. Chem. 2014, 53, 8120–8128. [Google Scholar] [CrossRef]
- Szczepanik, W.; Mlynarz, P.; Stefanowicz, P.; Kucharczyk-Klaminska, M.; D’Amelio, N.; Olbert-Majkut, A.; Staszewska, A.; Ratajska, M.; Szewczuk, Z.; Jezowska-Bojczuk, M. Structural studies of Cu(II) binding to the novel peptidyl derivative of quinoxaline: N-(3-(2,3-di(pyridin-2-yl)quinoxalin-6-yl)alanyl)glycine. Polyhedron 2011, 30, 9–15. [Google Scholar] [CrossRef]
- Milewska, M.; Guzow, K.; Wiczk, W. Fluorescent chemosensors for metal ions based on 3-(2-benzoxazol-5-yl)alanine skeleton. Open Chem. 2010, 8, 674–686. [Google Scholar] [CrossRef]
- Salmain, M.; Fischer-Durand, N.; Rudolf, B. Bioorthogonal Conjugation of Transition Organometallic Complexes to Peptides and Proteins: Strategies and Applications. Eur. J. Inorg. Chem. 2020, 2020, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Splith, K.; Neundorf, I.; Hu, W.; Peindy N’Dongo, H.W.; Vasylyeva, V.; Merz, K.; Schatzschneider, U. Influence of the metal complex-to-peptide linker on the synthesis and properties of bioactive CpMn(CO)3 peptide conjugates. Dalton Trans. 2010, 39, 2536–2545. [Google Scholar] [CrossRef]
- Salmain, M.; Fischer-Durand, N.; Cavalier, L.; Rudolf, B.; Zakrzewski, J.; Jaouen, G. Transition metal-carbonyl labeling of biotin and avidin for use in solid-phase carbonyl metallo immunoassay (CMIA). Bioconjug. Chem. 2002, 13, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.C. Artificial Metalloenzymes and Metallopeptide Catalysts for Organic Synthesis. ACS Catal. 2013, 3, 2954–2975. [Google Scholar] [CrossRef]
- Brewster, R.C.; Labeaga, I.C.; Soden, C.E.; Jarvis, A.G. Macrocylases as synthetic tools for ligand synthesis: Enzymatic synthesis of cyclic peptides containing metal-binding amino acids. R. Soc. Open Sci. 2021, 8, 211098. [Google Scholar] [CrossRef] [PubMed]
- Metrano, A.J.; Chinn, A.J.; Shugrue, C.R.; Stone, E.A.; Kim, B.; Miller, S.J. Asymmetric Catalysis Mediated by Synthetic Peptides, Version 2.0: Expansion of Scope and Mechanisms. Chem. Rev. 2020, 120, 11479–11615. [Google Scholar] [CrossRef]
- Guillena, G.; Halkes, K.M.; Rodríguez, G.; Batema, G.D.; van Koten, G.; Kamerling, J.P. Organoplatinum(II) complexes as a color biomarker in solid-phase peptide chemistry and screening. Org. Lett. 2003, 5, 2021–2024. [Google Scholar] [CrossRef] [PubMed]
- Dognini, P.; Coxon, C.R.; Alves, W.A.; Giuntini, F. Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art. Molecules 2021, 26, 693. [Google Scholar] [CrossRef]
- Shen, H.; Liu, J.; Lei, J.; Ju, H. A core–shell nanoparticle–peptide@metal–organic framework as pH and enzyme dual-recognition switch for stepwise-responsive imaging in living cells. Chem. Commun. 2018, 54, 9155. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, D.J.; Koksch, B. Peptide–Gold Nanoparticle Conjugates as Artificial Carbonic Anhydrase Mimics. Catalysts 2019, 9, 903. [Google Scholar] [CrossRef] [Green Version]
- Molev, G.; Lu, Y.; Kim, K.S.; Majdalani, I.C.; Guerin, G.; Petrov, S.; Walker, G.; Manners, I.; Winnik, M.A. Organometallic-Polypeptide Diblock Copolymers: Synthesis by DielsAlder Coupling and Crystallization-Driven Self-Assembly to Uniform Truncated Elliptical Lamellae. Macromolecules 2014, 47, 2604–2615. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Boyd, B.J. Peptide-based biosensors. Talanta 2015, 136, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei Garakani, T.; Sauer, D.F.; Mertens, M.A.S.; Lazar, J.; Gehrmann, J.; Arlt, M.; Schiffels, J.; Schnakenberg, U.; Okuda, J.; Schwaneberg, U. FhuA–Grubbs–Hoveyda biohybrid catalyst embedded in a polymer film enables catalysis in neat substrates. ACS Catalysis 2020, 10, 10946–10953. [Google Scholar] [CrossRef]
- Perera, A.S.; Subbaiyan, N.K.; Kalita, M.; Wendel, S.O.; Samarakoon, T.N.; D’Souza, F.; Bossmann, S.H. A hybrid soft solar cell based on the mycobacterial porin MspA linked to a sensitizer-viologen Diad. J. Am. Chem. Soc. 2013, 135, 6842–6845. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Zhang, S.; Zhao, P.; Wang, J.; Yan, M.; Ge, S.; Yu, J. Peptide cleavage-mediated photoelectrochemical signal on-off via CuS electronic extinguisher for PSA detection. Biosens. Bioelectron. 2020, 150, 111958. [Google Scholar] [CrossRef]
- Oh, K.J.; Cash, K.J.; Hugenberg, V.; Plaxco, K.W. Peptide beacons: A new design for polypeptide-based optical biosensors. Bioconjug. Chem. 2007, 18, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, R.; Paluch, A.; Rut, W.; Drąg, M.; Szewczuk, Z. On-bead Analysis of Substrate Specificity of Caspases using Peptide Modified by Qauternary Ammonium Group as Ionization Enhancers. J. Pept. Sci. 2018, 24, S132. [Google Scholar]
- Qin, J.-X.; Yang, X.-G.; Lv, C.-F.; Li, Y.-Z.; Liu, K.-K.; Zang, J.-H.; Yang, X.; Dong, L.; Shan, C.-X. Nanodiamonds: Synthesis, properties, and applications in nanomedicine. Mater. Des. 2021, 210, 110091. [Google Scholar] [CrossRef]
- Gaurav, I.; Wang, X.; Thakur, A.; Iyaswamy, A.; Thakur, S.; Chen, X.; Kumar, G.; Li, M.; Yang, Z. Peptide-Conjugated Nano Delivery Systems for Therapy and Diagnosis of Cancer. Pharmaceutics 2021, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef] [PubMed]
- Czarniewska, E.; Nowicki, P.; Kuczer, M.; Schroeder, G. Impairment of the immune response after transcuticular introduction of the insect gonadoinhibitory and hemocytotoxic peptide Neb-colloostatin: A nanotech approach for pest control. Sci. Rep. 2019, 9, 10330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Ahn, J.; Jung, S.; Lee, J.; Kang, T.; Jeong, J. Biomimetic Nanopillar-Based Biosensor for Label-Free Detection of Influenza A Virus. Biochip J. 2021, 15, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Guo, J.; Prokai, L. Selective chemoprecipitation and subsequent release of tagged species for the analysis of nitropeptides by liquid chromatography-tandem mass spectrometry. Mol. Cell. Proteom. 2011, 10, M110.002923. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Chi, B.; Wu, F.; Ma, S.; Zhan, S.; Yi, M.; Xu, H.; Mao, C. A sensitive label-free immunosensor for detection α-Fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens. Bioelectron. 2017, 95, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, M. Recent Progress in Electrochemical Immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S. Recent Advances in Electrochemical Immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent Advances in Electrochemical Immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef]
- Li, X.; Jian, M.; Sun, Y.; Zhu, Q.; Wang, Z. The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications. Molecules 2021, 26, 3228. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neely, A.; Perry, C.; Varisli, B.; Singh, A.K.; Arbneshi, T.; Senapati, D.; Kalluri, J.R.; Ray, P.C. Ultrasensitive and highly selective detection of Alzheimer’s disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano 2009, 3, 2834–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Chen, C.P.; Lee, C.H.; Chen, C.Y.; Lin, C.W. Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and a peptide aptamer. Chem. Commun. 2014, 50, 14443–14446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfragano, P.S.; Moro, G.; Polo, F.; Palchetti, I. The Role of Peptides in the Design of Electrochemical Biosensors for Clinical Diagnostics. Biosensors 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ha, K.; Guk, K.; Hwang, S.G.; Choi, J.M.; Kang, T.; Bae, P.; Jung, J.; Lim, E.K. Colorimetric detection of influenza A (H1N1) virus by a peptide-functionalized polydiacetylene (PEP-PDA) nanosensor. RSC Adv. 2016, 6, 48566–48570. [Google Scholar] [CrossRef] [Green Version]
- Pazos, E.; Torrecilla, D.; Vázquez López, M.; Castedo, L.; Mascareñas, J.L.; Vidal, A.; Vázquez, M.E. Cyclin A probes by means of intermolecular sensitization of terbium-chelating peptides. J. Am. Chem. Soc. 2008, 130, 9652–9653. [Google Scholar] [CrossRef] [PubMed]
- Heaton, I.; Platt, M. Peptide Nanocarriers for Detection of Heavy Metal Ions Using Resistive Pulse Sensing. Anal. Chem. 2019, 91, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Liu, D.; Wang, Z. Gold nanoparticle-based colorimetric sensor for studying the interactions of beta-amyloid peptide with metallic ions. Talanta 2010, 80, 1626–1631. [Google Scholar] [CrossRef]
- Siepi, M.; Oliva, R.; Petraccone, L.; Del Vecchio, P.; Ricca, E.; Isticato, R.; Lanzilli, M.; Maglio, O.; Lombardi, A.; Leone, L.; et al. Fluorescent peptide dH3w: A sensor for environmental monitoring of mercury (II). PLoS ONE 2018, 13, e0204164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial Peptides and Proteins: From Nature’s Reservoir to the Laboratory and Beyond. Front. Chem. 2021, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-J.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide-Nanoparticle Conjugates: A next Generation of Diagnostic and Therapeutic Platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and Protein Nanoparticle Conjugates: Versatile Platforms for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, M.J.; Olmedo, I.; Hosta, L.; Guerrero, A.R.; Cruz, L.J.; Albericio, F. Peptides and Metallic Nanoparticles for Biomedical Applications. Nanomed 2007, 2, 287–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.R.; Avelino, K.Y.P.S.; Ribeiro, K.L.; Franco, O.L.; Oliveira, M.D.L.; Andrade, C.A.S. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front. Biosci.-Sch. 2016, 8, 129–142. [Google Scholar]
- Mohid, S.A.; Ghorai, A.; Ilyas, H.; Mroue, K.H.; Narayanan, G.; Sarkar, A.; Ray, S.K.; Biswas, K.; Bera, A.K.; Malmsten, M.; et al. Application of Tungsten Disulfide Quantum Dot-Conjugated Antimicrobial Peptides in Bio-Imaging and Antimicrobial Therapy. Colloids Surf. B Biointerfaces 2019, 176, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Malekkhaiat Häffner, S.; Malmsten, M. Interplay between Amphiphilic Peptides and Nanoparticles for Selective Membrane Destabilization and Antimicrobial Effects. Curr. Opin. Colloid Interface Sci. 2019, 44, 59–71. [Google Scholar] [CrossRef]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; de Backer, M.E.; Rip, J.; Nabuurs, R.J.A.; Gaillard, P.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. Enhanced Glutathione PEGylated Liposomal Brain Delivery of an Anti-Amyloid Single Domain Antibody Fragment in a Mouse Model for Alzheimer’s Disease. J. Control. Release Off. J. Control. Release Soc. 2015, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Zhao, Y.; Dong, X.; Zheng, J.; Sun, Y. Design of a Molecular Hybrid of Dual Peptide Inhibitors Coupled on AuNPs for Enhanced Inhibition of Amyloid β-Protein Aggregation and Cytotoxicity. Small 2017, 13, 160166. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Kuczer, M.; Schroeder, G.; Czarniewska, E. Disruption of insect immunity using analogs of the pleiotropic insect peptide hormone Neb-colloostatin: A nanotech approach for pest control II. Sci. Rep. 2021, 11, 9459. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Di, S.; Wang, L.; Li, Z. Recent advances in the synthesis and applications of graphene-polypeptide nanocomposites. J. Mat. Chem. B 2021, 9, 6521–6535. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, H. Construction and Potential Applications of Biosensors for Proteins in Clinical Laboratory Diagnosis. Sensors 2017, 17, 2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Analyte | Interaction Mode | Signal Output | Strategy | Signal Marker 1 | Signal Marker 2 | Reference |
|---|---|---|---|---|---|---|
| Trypsin Chymotrypsin | Cleavage | Mass spectrometry | Lanthanide-Code | Lanthanide ions | [96] | |
| Papain Cathepsin B | Cleavage | Fluorescence | Energy transfer | Benzoxazol-5-yl-alanine derivatives | Tyr NO2 | [97] |
| AspN Endoproteinase Chymotrypsin | Cleavage | Fluorescence | Self-quenching | Cy-5 or Fluorescein | [98] | |
| MMP-2 and MMP-9 | Cleavage | Fluorescence | Self-quenching | Dye Ls276 | [99] | |
| Aspartyl Proteinases | Cleavage | Fluorescence | Quenching | o-aminobenzoyl | DNPED | [100] |
| Renin | Cleavage | Fluorescence | Quenching | 1,5AEDANS | DABCYL | [101] |
| ICE | Cleavage | Fluorescence | Quenching | 1,5AEDANS | DABCYL | [102] |
| Cytomegalovirus protease | Cleavage | Fluorescence | Quenching | EDANS | DABCYL | [103] |
| Neprilysin and insulin- | Cleavage | Fluorescence | Quenching | Alexa-350 | DABCYL | [104] |
| degrading enzyme | ||||||
| Secretase and caspases | Cleavage | Fluorescence | Quenching | PPE | QSY-7 | [105] |
| Trypsin, chymotrypsin, proteinase K, and thermolys | Cleavage | Fluorescence | Quenching | FITC | Gold nanoparticles | [106] |
| PSA | Cleavage | Fluorescence | Quenching | FITC | Gold nanoparticles | [107] |
| Chymotrypsin Trypsin Thrombine Human neutrophil elastase Granzyme B | Cleavage | Fluorescence | Quenching | ACC | [41,86] | |
| Thermolysine Chymotrypsin | Cleavage | Fluorescence | DANS | [108] | ||
| Lipases Esterases | Cleavage | Fluorescene | Quenching | AMC | [109] | |
| Synchronous detection of trypsin and chymotrypsin | Cleavage | Fluorescence | Quenching | DEAC Rhodamine B | [110] | |
| Trypsin Chymotrypsin | Cleavage | Fluorescence | Quenching | DMOAP-coated glass slides | [111] | |
| Caspase-3 | Cleavage | Fluorescence | Quenching | FAM | Graphene oxide | [112] |
| Chymotrypsin and MMP-2 | Cleavage | Fluorescence | Quenching | Metalloprotoporp hyrins, QXL 570 | Graphene oxide | [113] |
| Thrombin | Cleavage | Fluorescence | Quenching | FAM | SWCNHs | [114] |
| Trypsin, chymotrypsin, V8 protease, plasmin, thrombin, and pepsin | Cleavage | Fluorescence | FRET | Mca-fluorophore | Dinitrophenyl | [113] |
| Prokaryotic enzyme | Cleavage | Fluorescence | FRET | FITC | Dabcyl (Dbc) | [115] |
| MMP-1 and MMP-9 | Cleavage | Fluorescence | FRET | 5FAM | Cy5 | [116] |
| HIV protease | Cleavage | Fluorescence | Quenching | EDANS | DABCYL | [117] |
| MMP-7 | Cleavage | NIR fluorescence | FRET | Cy5.5 | NIR8Q20 | [118] |
| Thrombin | Cleavage | NIR fluorescence | Self-quenching | Cy 5.5 | [119] | |
| Tumor protease | Cleavage | NIR fluorescence | Self-quenching | Cy 5.5 | [120] | |
| PSA | Cleavage | Current | Electrochemical | Ferrocene | [121] | |
| Trypsin and α-Thrombin | Cleavage | Current | Electrochemical | Ferrocene | [122] | |
| MMP-7 | Cleavage | Color density | Silver enhancement on Au particle | Au particle | [123] | |
| PSA | Cleavage | Luminescence | Electrogenerated luminescence | Ru (II) chelate | [124] | |
| PSA | Cleavage | Luminescence | Electrogenerated luminescence | Ru (II) chelate | Ferrocene | [125] |
| Trypsin Chymotrypsin Caspases | Cleavage | Mass spectrometry | Fixed-charge tag-coded | Fixed-charge tagged ions | [126,127,128,129] |
| Analyte | Interaction Mode | Signal Output | Strategy | Signal Marker 1 | Reference |
|---|---|---|---|---|---|
| H1N1 virus | Hemagglutinin affinity | Reflectance | Nanopillar optics | Refractive index | [174] |
| H1N1 virus | Peptide affinity | Colorimetry | Conformation shift | Polydiacetylene nanoparticle | [185] |
| Cyclin A | Cationic probe affinity | Colorimetry | nanoparticle aggregation | Au nanoparticle | [106] |
| Cyclin A | grove recognition | Fluorescence | intermolecular sensitization | Tb3+ chelating macrocycle DOTA/Trp | [186] |
| Anti-HIV antibodies | Epitope recognition | Fluorescence | Energy transfer | Fluorophore/quencher | [168] |
| Prostate specific antigen | Epitope recognition | Photoelectrochemistry | Peptide cleavage | Signal amplification | [167] |
| α-Fetoprotein | Epitope recognition | Electrochemistry | Immunoprecipitation | Amperometric response | [176] |
| Alzheimer’s tau protein | Epitope recognition | Two-photon Rayleigh scattering, Colorimetry | Chromophore shape | λmax shift | [182] |
| Gonadotropin | Aptamer affinity | Colorimetry | Redox catalysis | 4NP/4AP | [183] |
| Nitropeptides | Chemoprecipitation | Mass spectrometry | Capture and labeling | Mass tag | [175] |
| Ni ions | Coordination | Resistive pulse sensing | His tag | Translocation velocity | [187] |
| Zn ions | Aggregation | Colorimetry | Aggregation | Au nanoparticle | [188] |
| Hg, Cu and Zn ions | Coordination | Fluorescence | FRET | Dansyl/Trp | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, M.; Popiel, D.; Walter, M.; Bąchor, R.; Biernat, M.; Cebrat, M.; Kijewska, M.; Kuczer, M.; Modzel, M.; Kluczyk, A. Veni, Vidi, Vici: Immobilized Peptide-Based Conjugates as Tools for Capture, Analysis, and Transformation. Chemosensors 2022, 10, 31. https://doi.org/10.3390/chemosensors10010031
Kowalska M, Popiel D, Walter M, Bąchor R, Biernat M, Cebrat M, Kijewska M, Kuczer M, Modzel M, Kluczyk A. Veni, Vidi, Vici: Immobilized Peptide-Based Conjugates as Tools for Capture, Analysis, and Transformation. Chemosensors. 2022; 10(1):31. https://doi.org/10.3390/chemosensors10010031
Chicago/Turabian StyleKowalska, Marta, Dominik Popiel, Martyna Walter, Remigiusz Bąchor, Monika Biernat, Marek Cebrat, Monika Kijewska, Mariola Kuczer, Maciej Modzel, and Alicja Kluczyk. 2022. "Veni, Vidi, Vici: Immobilized Peptide-Based Conjugates as Tools for Capture, Analysis, and Transformation" Chemosensors 10, no. 1: 31. https://doi.org/10.3390/chemosensors10010031
APA StyleKowalska, M., Popiel, D., Walter, M., Bąchor, R., Biernat, M., Cebrat, M., Kijewska, M., Kuczer, M., Modzel, M., & Kluczyk, A. (2022). Veni, Vidi, Vici: Immobilized Peptide-Based Conjugates as Tools for Capture, Analysis, and Transformation. Chemosensors, 10(1), 31. https://doi.org/10.3390/chemosensors10010031