Wax-Printed Fluidic Controls for Delaying and Accelerating Fluid Transport on Paper-Based Analytical Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Paper-Based Devices with Wax-Printed Delays and Accelerators
2.2. Fluid Flow Rate Experiments
2.3. Paper-Based Kinetic Enzymatic Assay
3. Results and Discussion
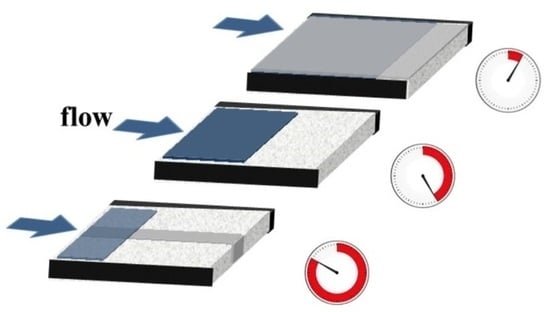
3.1. Hydrophobic Fluidic Delays for Delaying Fluid Transport
3.2. Hydrophobic Overlays for Accelerating Fluid Transport
3.3. Combined Use of Fluidic Delays and Accelerators
3.4. Comparison with Similar Methods
3.5. Paper-Based Kinetic Enzymatic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in paper-based analytical devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Bui, M.-P.N.; Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosens. Bioelectron. 2016, 77, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.G.S.; Jayawardane, B.M.; Kolev, S.D.; McKelvie, I.D. Developments of microfluidic paper-based analytical devices (μPADs) for water analysis: A review. Talanta 2018, 177, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Noh, H.; Phillips, S.T. Metering the Capillary-Driven Flow of Fluids in Paper-Based Microfluidic Devices. Anal. Chem. 2010, 82, 4181–4187. [Google Scholar] [CrossRef]
- Vella, S.J.; Beattie, P.; Cademartiri, R.; Laromaine, A.; Martinez, A.W.; Phillips, S.T.; Mirica, K.A.; Whitesides, G.M. Measuring Markers of Liver Function Using a Micropatterned Paper Device Designed for Blood from a Fingerstick. Anal. Chem. 2012, 84, 2883–2891. [Google Scholar] [CrossRef] [Green Version]
- Lyu, X.; Hamedpour, V.; Sasaki, Y.; Zhang, Z.; Minami, T. 96-Well Microtiter Plate Made of Paper: A Printed Chemosensor Array for Quantitative Detection of Saccharides. Anal. Chem. 2021, 93, 1179–1184. [Google Scholar] [CrossRef]
- Kappi, F.A.; Tsogas, G.Z.; Routsi, A.-M.; Christodouleas, D.C.; Giokas, D.L. Paper-based devices for biothiols sensing using the photochemical reduction of silver halides. Anal. Chim. Acta 2018, 1036, 89–96. [Google Scholar] [CrossRef]
- Kappi, F.A.; Tsogas, G.Z.; Christodouleas, D.C.; Giokas, D.L. Calibrant-loaded paper-based analytical devices for standard addition quantitative assays. Sens. Actuators B Chem. 2017, 253, 860–867. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yang, J.-T. Detection of an amphiphilic biosample in a paper microchannel based on length. Biomed. Microdevices 2015, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Apilux, A.; Ukita, Y.; Chikae, M.; Chailapakul, O.; Takamura, Y. Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab Chip 2013, 13, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Anderson, M.J.; Crooks, R.M. Electrochemistry in hollow-channel paper analytical devices. J. Am. Chem. Soc. 2014, 136, 4616–4623. [Google Scholar] [CrossRef]
- Kokkinos, C.T.; Giokas, D.L.; Economou, A.S.; Petrou, P.S.; Kakabakos, S.E. Paper-based microfluidic device with integrated sputtered electrodes for stripping voltammetric determination of DNA via quantum dot labeling. Anal. Chem. 2018, 90, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Downs, C. Progress in the development and integration of fluid flow control tools in paper microfluidics. Lab Chip 2017, 17, 614–628. [Google Scholar] [CrossRef]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialisation. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef]
- Giokas, D.L.; Tsogas, G.Z.; Vlessidis, A.G. Programming fluid transport in paper-based microfluidic devices using razor-crafted open channels. Anal. Chem. 2014, 86, 6202–6207. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fluid transport mechanisms in paper-based microfluidic devices. In Paper Microfluidics-Theory and Applications, 1st ed.; Bhattacharya, S., Kumar, S., Agarwal, K.A., Eds.; Springer: Singapore, 2019; pp. 7–28. [Google Scholar]
- Fu, E.; Ramsey, S.A.; Kauffman, P.; Lutz, B.; Yager, P. Transport in two-dimensional paper networks. Microfluid. Nanofluidics 2011, 10, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Jang, I.; Song, S. Facile and precise flow control for a paper-based microfluidic device through varying paper permeability. Lab Chip 2015, 15, 3405–3412. [Google Scholar] [CrossRef]
- Noh, H.; Phillips, S.T. Fluidic timers for time-dependent, point-of-care assays on paper. Anal. Chem. 2010, 82, 8071–8078. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-H.; Chen, M.-Y.; Shen, C.-H.; Yang, R.-J. Colored wax-printed timers for two-dimensional and three-dimensional assays on paper-based devices. Biomicrofluidics 2014, 8, 066502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Kim, W. Dynamics of water imbibition through paper channels with wax boundaries. Microfluid. Nanofluidics 2015, 19, 845–853. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarara, M.; Giokas, D.L.; Tsogas, G.Z. Wax-Printed Fluidic Controls for Delaying and Accelerating Fluid Transport on Paper-Based Analytical Devices. Chemosensors 2022, 10, 155. https://doi.org/10.3390/chemosensors10050155
Tarara M, Giokas DL, Tsogas GZ. Wax-Printed Fluidic Controls for Delaying and Accelerating Fluid Transport on Paper-Based Analytical Devices. Chemosensors. 2022; 10(5):155. https://doi.org/10.3390/chemosensors10050155
Chicago/Turabian StyleTarara, Maria, Dimosthenis L. Giokas, and George Z. Tsogas. 2022. "Wax-Printed Fluidic Controls for Delaying and Accelerating Fluid Transport on Paper-Based Analytical Devices" Chemosensors 10, no. 5: 155. https://doi.org/10.3390/chemosensors10050155
APA StyleTarara, M., Giokas, D. L., & Tsogas, G. Z. (2022). Wax-Printed Fluidic Controls for Delaying and Accelerating Fluid Transport on Paper-Based Analytical Devices. Chemosensors, 10(5), 155. https://doi.org/10.3390/chemosensors10050155