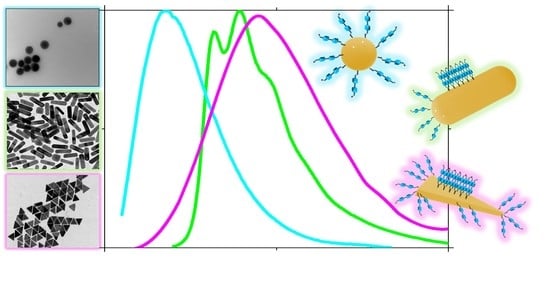
Luminescent Self-Assembled Monolayer on Gold Nanoparticles: Tuning of Emission According to the Surface Curvature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Gold Nanorods
2.2. Synthesis of Gold Nanospheres
2.3. Synthesis of Gold Nanotriangles
2.4. Bpy-SH Covered Gold Plates
3. Results and Discussion
3.1. Uv-Vis Spectroscopy
3.2. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F. Structure and Growth of Self-Assembling Monolayers. Prog. Surf. Sci. 2000, 65, 151–257. [Google Scholar] [CrossRef]
- Kühnle, A. Self-Assembly of Organic Molecules at Metal Surfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 157–168. [Google Scholar] [CrossRef]
- Chaki, N.K.; Vijayamohanan, K. Self-Assembled Monolayers as a Tunable Platform for Biosensor Applications. Biosens. Bioelectron. 2002, 17, 1–12. [Google Scholar] [CrossRef]
- Motealleh, A.; Dorri, P.; Kehr, N.S. Self-Assembled Monolayers of Chiral Periodic Mesoporous Organosilica as a Stimuli Responsive Local Drug Delivery System. J. Mater. Chem. B 2019, 7, 2362–2371. [Google Scholar] [CrossRef]
- Gankin, A.; Mervinetsky, E.; Alshanski, I.; Buchwald, J.; Dianat, A.; Gutierrez, R.; Cuniberti, G.; Sfez, R.; Yitzchaik, S. ITO Work Function Tunability by Polarizable Chromophore Monolayers. Langmuir 2019, 35, 2997–3004. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M.; Allara, D.L.; Tao, Y.T.; Parikh, A.N.; Nuzzo, R.G. Comparison of the Structures and Wetting Properties of Self-Assembled Monolayers of n-Alkanethiols on the Coinage Metal Surfaces, Copper, Silver, and Gold. J. Am. Chem. Soc. 1991, 113, 7152–7167. [Google Scholar] [CrossRef]
- Heister, K.; Allara, D.L.; Bahnck, K.; Frey, S.; Zharnikov, M.; Grunze, M. Deviations from 1:1 Compositions in Self-Assembled Monolayers Formed from Adsorption of Asymmetric Dialkyl Disulfides on Gold. Langmuir 1999, 15, 5440–5443. [Google Scholar] [CrossRef]
- Hamoudi, H.; Esaulov, V.A. Selfassembly of α,ω-Dithiols on Surfaces and Metal Dithiol Heterostructures. Ann. Phys. 2016, 528, 242–263. [Google Scholar] [CrossRef] [Green Version]
- Hamoudi, H. Bottom-up Nanoarchitectonics of Two-Dimensional Freestanding Metal Doped Carbon Nanosheet. RSC Adv. 2014, 4, 22035–22041. [Google Scholar] [CrossRef]
- Hamoudi, H.; Prato, M.; Dablemont, C.; Cavalleri, O.; Canepa, M.; Esaulov, V.A. Self-Assembly of 1,4-Benzenedimethanethiol Self-Assembled Monolayers on Gold. Langmuir 2010, 26, 7242–7247. [Google Scholar] [CrossRef] [PubMed]
- Pethkar, S.; Aslam, M.; Mulla, I.S.; Ganeshan, P.; Vijayamohanan, K. Preparation and Characterisation of Silver Quantum Dot Superlattice Using Self-Assembled Monolayers of Pentanedithiol. J. Mater. Chem. 2001, 11, 1710–1714. [Google Scholar] [CrossRef]
- Sarathy, K.V.; Thomas, P.J.; Kulkarni, G.U.; Rao, C.N.R. Superlattices of Metal and Metal-Semiconductor Quantum Dots Obtained by Layer-by-Layer Deposition of Nanoparticle Arrays. J. Phys. Chem. B 1999, 103, 399–401. [Google Scholar] [CrossRef]
- Jackson, A.M.; Myerson, J.W.; Stellacci, F. Spontaneous Assembly of Subnanometre-Ordered Domains in the Ligand Shell of Monolayer-Protected Nanoparticles. Nat. Mater. 2004, 3, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Lu, W.; Suo, Z. Symmetry Breaking in Self-Assembled Monolayers on Solid Surfaces: Anisotropic Surface Stress. Phys. Rev. B 2002, 65, 085401. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold–sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Liang, J.; Rosa, L.G.; Scoles, G. Nanostructuring, Imaging and Molecular Manipulation of Dithiol Monolayers on Au(111) Surfaces by Atomic Force Microscopy. J. Phys. Chem. C 2007, 111, 17275–17284. [Google Scholar] [CrossRef]
- Rieley, H.; Kendall, G.K.; Zemicael, F.W.; Smith, T.L.; Yang, S. X-ray Studies of Self-Assembled Monolayers on Coinage Metals. 1. Alignment and Photooxidation in 1,8-Octanedithiol and 1-Octanethiol on Au. Langmuir 1998, 14, 5147–5153. [Google Scholar] [CrossRef]
- De Luca, O.; Caruso, T.; Turano, M.; Ionescu, A.; Godbert, N.; Aiello, I.; Ghedini, M.; Formoso, V.; Agostino, R.G. Adsorption of Nile Red Self-Assembled Monolayers on Au (111). Langmuir 2019, 35, 14761–14768. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, F.; Ionescu, A.; Aiello, I.; La Deda, M.; Crispini, A.; Ghedini, M.; Brunelli, E.; Sesti, S.; Godbert, N. High Order in a Self-Assembled Iridium(III) Complex Gelator Towards Nanostructured IrO2 Thin Films. Chem.-Asian J. 2018, 12, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Lu, D.; Tian, W.; Zhang, R.; Yu, H.; Gorecka, E.; Pociecha, D.; Godbert, N.; Hao, J.; Li, H. Ordered structures of alkylated carbon dots and their applications in nonlinear optics. J. Mater. Chem. C 2020, 8, 8980–8991. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Wang, Y.; Quan, Y.; Chen, D.; Cheng, Y. Strong Aggregation-Induced CPL Response Promoted by Chiral Emissive Nematic Liquid Crystals (N*-LCs). Chem. Eur. J. 2018, 24, 12607–12612. [Google Scholar] [CrossRef]
- Galyametdinov, Y.G.; Knyazev, A.A.; Dzhabarov, V.I.; Cardinaels, T.; Driesen, K.; Görller-Walrand, C.; Binnemans, K. Polarized Luminescence from Aligned Samples of Nematogenic Lanthanide Complexes. Adv. Mater. 2008, 20, 252–257. [Google Scholar] [CrossRef]
- Agina, E.V.; Mannanov, A.A.; Sizov, A.S.; Vechter, O.; Borshchev, O.V.; Bakirov, A.V.; Shcherbina, M.A.; Chvalun, S.N.; Konstantinov, V.G.; Bruevich, V.V.; et al. Luminescent Organic Semiconducting Langmuir Monolayers. ACS Appl. Mater. Interfaces 2017, 9, 18078–18086. [Google Scholar] [CrossRef]
- Yokota, H.; Okazaki, K.; Shimura, K.; Nakayama, M.; Kim, D. Photoluminescence Properties of Self-Assembled Monolayers of CdSe and CdSe/ZnS Quantum Dots. J. Phys. Chem. C 2012, 116, 5456–5459. [Google Scholar] [CrossRef]
- Séro, L.; Sanguinet, L.; Derbré, S.; Boury, F.; Brotons, G.; Dabos-Seignon, S.; Richomme, P.; Séraphin, D. Fluorescent Self-Assembled Monolayers of Umbelliferone: A Relationship between Contact Angle and Fluorescence. Langmuir 2013, 29, 10423–10431. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-S.; Lees, A.J. Photophysics and Evidence of Excimer Formation, Linear Bipyridines in Solution and Solid Films. J. Photochem. Photobiol. A 2001, 140, 157–161. [Google Scholar] [CrossRef]
- Scarabelli, L.; Grzelczak, M.; Liz-Marzán, L.M. Tuning Gold Nanorod Synthesis through Prereduction with Salicylic Acid. Chem. Mater. 2013, 25, 4232–4238. [Google Scholar] [CrossRef] [Green Version]
- Candreva, A.; Lewandowski, W.; La Deda, M. Thickness control of the silica shell: A way to tune the plasmonic properties of isolated and assembled gold nanorods. J. Nanopart. Res. 2021, 24, 19. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, Y.; Smalyukh, I.I. Electrically and Optically Tunable Plasmonic Guest–Host Liquid Crystals with Long-Range Ordered Nanoparticles. Nano Lett. 2014, 14, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, L.; Coronado-Puchau, M.; Giner-Casares, J.J.; Langer, J.; Liz-Marzán, L.M. Monodisperse Gold Nanotriangles: Size Control, Large-Scale Self-Assembly, and Performance in Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- Sakotsubo, Y.; Ohgi, T.; Fujita, D.; Ootuka, Y. Tunneling Spectroscopy of Isolated Gold Clusters Grown on Thiol/Dithiol Mixed Self-Assembled Monolayers. Phys. E Low-Dimens. Syst. Nanostruct. 2005, 29, 601–605. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 Nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Gao, M.; Zheng, X.; Khan, I.; Cai, H.; Lan, J.; Liu, J.; Wang, J.; Wu, J.; Huang, S.; Li, S.; et al. Resonant Light Absorption and Plasmon Tunability of Lateral Triangular Au Nanoprisms Array. Phys. Lett. A 2019, 383, 125881. [Google Scholar] [CrossRef]
- Battistini, G.; Cozzi, P.G.; Jalkanen, J.-P.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Zerbetto, F. The Erratic Emission of Pyrene on Gold Nanoparticles. ACS Nano 2008, 2, 77–84. [Google Scholar] [CrossRef]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef] [Green Version]
- Candreva, A.; Di Maio, G.; La Deda, M. A Quick One-Step Synthesis of Luminescent Gold Nanospheres. Soft Matt. 2020, 16, 10865–10868. [Google Scholar] [CrossRef]
- De Luca, A.; Ferrie, M.; Ravaine, S.; La Deda, M.; Infusino, M.; Rashed, A.R.; Veltri, A.; Aradian, A.; Scaramuzza, N.; Strangi, G. Gain Functionalized Core-Shell Nanoparticles: The Way to Selectively Compensate Absorptive Losses. J. Mater. Chem. 2012, 22, 8846–8852. [Google Scholar] [CrossRef]
- Scarpelli, F.; Ionescu, A.; Ricciardi, L.; Plastina, P.; Aiello, I.; la Deda, M.; Crispini, A.; Ghedini, M.; Godbert, N. A novel route towards water-soluble luminescent iridium(III) complexes via a hydroxy-bridged dinuclear precursor. Dalton Trans. 2016, 45, 17264–17273. [Google Scholar] [CrossRef] [PubMed]
- Liguori, P.F.; Ghedini, M.; la Deda, M.; Godbert, N.; Parisi, F.; Guzzi, R.; Ionescu, A.; Aiello, I. Electrochromic behaviour of Ir(III) bis-cyclometalated 1,2-dioxolene tetra-halo complexes: Fully reversible catecholate/semiquinone redox switches. Dalton Trans. 2020, 49, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Caligiuri, R.; Godbert, N.; Candreva, A.; la Deda, M.; Furia, E.; Ghedini, M.; Aiello, I. New electropolymerizable Ir(III) complexes with β-ketoiminate ancillary ligands. Chem. Asian J. 2019, 14, 3025–3034. [Google Scholar] [CrossRef]
- Mutai, T.; Cheon, J.D.; Tsuchiya, G.; Araki, K. Tuning of fluorescence properties of aminopyridine*** fluorophores by N-substitution. J. Chem. Soc. Perkin Trans. 2 2002, 2, 862–865. [Google Scholar] [CrossRef]
- Poizat, O.; Buntinx, G.; Valat, P.; Wintgens, V.; Bridoux, M. Photochemistry of 4,4′-Bipyridine. Nanosecond Absorption and Raman Study of the Hydrogen Atom Abstraction from Methanol and 2-Propanol. J. Phys. Chem. 1993, 97, 5905–5910. [Google Scholar] [CrossRef]
- Gao, X. Reproducibility in Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. 1990, 94, 6858–6864. [Google Scholar] [CrossRef]
- Ducasse, L.; Dussauze, M.; Grondin, J.; Lassègues, J.C.; Naudin, C.; Servant, L. Spectroscopic study of poly(ethylene oxide)6: LiX complexes (X = PF6, AsF6, SbF6, ClO4). Phys. Chem. Chem. Phys. 2003, 5, 567–574. [Google Scholar] [CrossRef]
- Gupta, R.; Dyer, M.J.; Weimer, W.A. Preparation and characterization of surface plasmon resonance tunable gold and silver films. J. Appl. Phys. 2002, 92, 5264–5271. [Google Scholar] [CrossRef]
- Yang, T.; Long, H.; Malkoch, M.; Gamstedt, E.K.; Berglund, L.; Hult, A. Characterization of Well-Defined Poly(ethylene glycol) Hydrogels Prepared by Thiol-ene Chemistry. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4044–4054. [Google Scholar] [CrossRef]
- Ould-Moussa, L.; Castella-Ventura, M.; Kassab, E.; Poizat, O.; Strommen, D.P.; Kincaid, J.R. Ab initio and density functional study of the geometrical, electronic and vibrational properties of 2,2′-bipyridine. J. Raman Spectrosc. 2000, 31, 377–390. [Google Scholar] [CrossRef]
- Kumar, J.; Thomas, R.; Swathi, R.S.; Thomas, K.G. Au nanorod quartets and Raman signal enhancement: Towards the design of plasmonic platforms. Nanoscale 2014, 6, 10454–10459. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.W.; Han, S.W.; Kim, K. Adsorption Characteristics of p-Xylene-r,r′-dithiol on Gold and Silver Surfaces: Surface-Enhanced Raman Scattering and Ellipsometry Study. J. Phys. Chem. B 1999, 103, 10831–10837. [Google Scholar] [CrossRef]
- Kumar, J.; Thomas, K.G. Surface-Enhanced Raman Spectroscopy: Investigations at the Nanorod Edges and Dimer Junctions. J. Phys. Chem. Lett. 2011, 2, 610–615. [Google Scholar] [CrossRef]
- Kertesz, M.; Choi, C.H.; Hong, S.Y. Conformational Information from Vibrational Spectra of Polyaniline. Synth. Met. 1997, 85, 1073–1076. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, M. Fast loading of PEG–SH on CTAB-protected gold nanorods. RSC Adv. 2014, 4, 17760–17767. [Google Scholar] [CrossRef]
- Serrano-Montes, A.B.; Jimenez de Aberasturi, D.; Langer, J.; Giner-Casares, J.J.; Scarabelli, L.; Herrero, A.; Liz-Marzán, L.M. A General Method for Solvent Exchange of Plasmonic Nanoparticles and Self-Assembly into SERS-Active Monolayers. Langmuir 2015, 31, 9205–9213. [Google Scholar] [CrossRef] [Green Version]
- Yüce, M.; Kurt, H. How to make nanobiosensors: Surface modification and characterisation of nanomaterials for biosensing applications. RSC Adv. 2017, 7, 49386–49403. [Google Scholar] [CrossRef] [Green Version]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Whitney, A.V.; van Duyne, R.P.; Casadio, F. An innovative surface-enhanced Raman spectroscopy (SERS) method for the identification of six historical red lakes and dyestuffs. J. Raman Spectrosc. 2006, 37, 993–1002. [Google Scholar] [CrossRef]













| Sample | Abs, λ/nm (ε/M−1 cm−1) | Emission Max, λ/nm | Excitation Bands, λ/nm | Lifetime, τ/ns (α/%) | |
|---|---|---|---|---|---|
| at 352 nm | at 418 nm | ||||
| Bpy-SH@AuNR a | 273, 300(sh),368, 390(sh), 530, 840 c | 435 | 320, 374, 395(sh) | 1.6 (94.3), 11.1 (5.7) | 0.7 (75.4), 5.2 (24.6) |
| Bpy-SH@AuNS a | 291, 520 c | 350 | 265 | 1.5 (79.3), 7.6 (20.7) | |
| Bpy-SH@AuNT a | 294, 350(sh), 653 c | 410 | 265, 382 | 0.7 (82.5), 2.4 (17.5) | |
| “diluted” Bpy-SH solution (1.3 × 10−5 M) b | 254 (15145), 295 (29355), 310 (20300, sh), 330 (1470, sh) | 356 | 270 | 2.2 (40.2), 7.9 (59.8) | |
| “concentrated” Bpy-SH solution (3.5 × 10−2 M) b | Too intense | 418 | 362 | 0.9 (81.5), 3.0 (18.5) | |
| Solid Bpy-SH | Not available | 435 | 397 | 0.7 (86.0), 2.7 (14.0) | |
| Bpy-SH@Au-plate | Not available | 435 | 280, 361 | 0.6 (5.5), 0.9 (94.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candreva, A.; Di Maio, G.; Parisi, F.; Scarpelli, F.; Crispini, A.; Godbert, N.; Ricciardi, L.; Nucera, A.; Rizzuto, C.; Barberi, R.C.; et al. Luminescent Self-Assembled Monolayer on Gold Nanoparticles: Tuning of Emission According to the Surface Curvature. Chemosensors 2022, 10, 176. https://doi.org/10.3390/chemosensors10050176
Candreva A, Di Maio G, Parisi F, Scarpelli F, Crispini A, Godbert N, Ricciardi L, Nucera A, Rizzuto C, Barberi RC, et al. Luminescent Self-Assembled Monolayer on Gold Nanoparticles: Tuning of Emission According to the Surface Curvature. Chemosensors. 2022; 10(5):176. https://doi.org/10.3390/chemosensors10050176
Chicago/Turabian StyleCandreva, Angela, Giuseppe Di Maio, Francesco Parisi, Francesca Scarpelli, Alessandra Crispini, Nicolas Godbert, Loredana Ricciardi, Antonello Nucera, Carmen Rizzuto, Riccardo C. Barberi, and et al. 2022. "Luminescent Self-Assembled Monolayer on Gold Nanoparticles: Tuning of Emission According to the Surface Curvature" Chemosensors 10, no. 5: 176. https://doi.org/10.3390/chemosensors10050176
APA StyleCandreva, A., Di Maio, G., Parisi, F., Scarpelli, F., Crispini, A., Godbert, N., Ricciardi, L., Nucera, A., Rizzuto, C., Barberi, R. C., Castriota, M., & La Deda, M. (2022). Luminescent Self-Assembled Monolayer on Gold Nanoparticles: Tuning of Emission According to the Surface Curvature. Chemosensors, 10(5), 176. https://doi.org/10.3390/chemosensors10050176