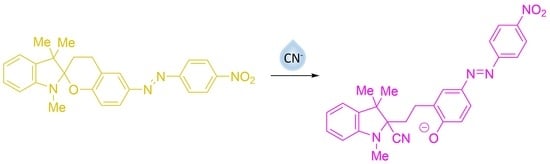
Cyanide Anion Determination Based on Nucleophilic Addition to 6-[(E)-(4-Nitrophenyl)diazenyl]-1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indole] Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.1.1. 4-[(E)-2-Chloro-4′-nitrophenyl]diazenyl-2-hydroxymethylphenol (3b)
2.1.2. General Procedure for Preparation of 4
2.1.3. General Procedure for Synthesis of 7
2.2. Analytical Procedure
3. Results and Discussion
3.1. Synthesis
3.2. UV–Vis Spectral Investigations and Chemosensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science, Taylor & Francis Group, LLC: New York, NY, USA, 2015; p. 772. [Google Scholar] [CrossRef]
- Aranguri-Llerena, G.; Siche, R. Superior Plants with Significant Amounts of Cyanide and Their Toxicological Implications. Rev. Agric. Sci. 2020, 8, 354–366. [Google Scholar] [CrossRef]
- Dolan, L.C.; Matulka, R.A.; Burdock, G.A. Naturally Occurring Food Toxins. Toxins 2010, 2, 2289–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson-Smith, P.; Jansen, E.C.; Hyldegaard, O. Cyanide intoxication as part of smoke inhalation-a review on diagnosis and treatment from the emergency perspective. Scand. J. Trauma Resusc. 2011, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumari, D. Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2016. Sens. Actuators B Chem. 2018, 259, 1022–1057. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S. On-site and low-cost detection of cyanide by simple colorimetric and fluorogenic sensors: Smartphone and test strip applications. Talanta 2020, 207, 120278. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gradzielski, M. Fluorescence sensing of cyanide anions based on Au-modified upconversion nanoassemblies. Analyst 2021, 146, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Isaad, J.; Perwuelz, A. New color chemosensors for cyanide based on water soluble azo dyes. Tetrahedron Lett. 2010, 51, 5810–5814. [Google Scholar] [CrossRef]
- Gong, W.T.; Zhang, Q.L.; Shang, L.; Gao, B.; Ning, G.L. A new principle for selective sensing cyanide anions based on 2-hydroxy-naphthaldeazine compound. Sens. Actuators B Chem. 2013, 177, 322–326. [Google Scholar] [CrossRef]
- Lee, D.Y.; Singh, N.; Satyender, A.; Jang, D.O. An azo dye-coupled tripodal chromogenic sensor for cyanide. Tetrahedron Lett. 2011, 52, 6919–6922. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, W.; Tian, H. A highly sensitive and selective chemosensor for cyanide. Talanta 2008, 75, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Adachi, K.; Itoh, M.; Hirai, T. Spiropyran as a Selective, Sensitive, and Reproducible Cyanide Anion Receptor. Org. Lett. 2009, 11, 3482–3485. [Google Scholar] [CrossRef] [PubMed]
- Orojloo, M.; Amani, S. Naked-eye detection of cyanide ions in aqueous media based on an azo-azomethine chemosensor. Comptes Rendus Chim. 2017, 20, 415–423. [Google Scholar] [CrossRef]
- Bhaskar, R.; Sarveswari, S. Colorimetric sensor for real-time detection of cyanide ion in water and food samples. Inorg. Chem. Commun. 2019, 102, 83–89. [Google Scholar] [CrossRef]
- Morikawa, Y.; Nishiwaki, K.; Suzuki, S.; Yasaka, S.; Okada, Y.; Nakanishi, I. A new chemosensor for cyanide in blood based on the Pd complex of 2-(5-bromo-2-pyridylazo)-5-[N-n-propyl-N-(3-sulfopropyl)amino]phenol. Analyst 2020, 145, 7759–7764. [Google Scholar] [CrossRef]
- Gai, L.; Mack, J.; Lu, H.; Nyokong, T.; Li, Z.; Kobayashi, N.; Shen, Z. Organosilicon compounds as fluorescent chemosensors for fluoride anion recognition. Coord. Chem. Rev. 2015, 285, 24–51. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, S.H. Colorimetric chemodosimeter for cyanide detection based on spiropyran derivative and its thermodynamic studies. Dyes Pigm. 2014, 102, 228–233. [Google Scholar] [CrossRef]
- Tomasulo, M.; Raymo, F.M. Colorimetric Detection of Cyanide with a Chromogenic Oxazine. Org. Lett. 2005, 7, 4633–4636. [Google Scholar] [CrossRef]
- Tomasulo, M.; Sortino, S.; White, A.J.P.; Raymo, F.M. Chromogenic Oxazines for Cyanide Detection. J. Org. Chem. 2006, 71, 744–753. [Google Scholar] [CrossRef]
- Shao, N.; Zhang, Y.; Cheung, S.; Yang, R.; Chan, W.; Mo, T.; Li, K.; Liu, F. Copper Ion-Selective Fluorescent Sensor Based on the Inner Filter Effect Using a Spiropyran Derivative. Anal. Chem. 2005, 77, 7294–7303. [Google Scholar] [CrossRef]
- Potisek, S.L.; Davis, D.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanophore-Linked Addition Polymers. J. Am. Chem. Soc. 2007, 129, 13808–13809. [Google Scholar] [CrossRef] [PubMed]
- Kiyose, K.; Hanaoka, K.; Oushiki, D.; Nakamura, T.; Kajimura, M.; Suematsu, M.; Nishimatsu, H.; Yamane, T.; Terai, T.; Hirata, Y.; et al. Hypoxia-Sensitive Fluorescent Probes for in Vivo Real-Time Fluorescence Imaging of Acute Ischemia. J. Am. Chem. Soc. 2010, 132, 15846–15848. [Google Scholar] [CrossRef] [PubMed]
- Dagilienė, M.; Martynaitis, V.; Vengris, M.; Redeckas, K.; Voiciuk, V.; Holzer, W.; Šačkus, A. Synthesis of 1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indoles] as a new class of ultrafast light-driven molecular switch. Tetrahedron 2013, 69, 9309–9315. [Google Scholar] [CrossRef]
- Dagilienė, M.; Martynaitis, V.; Kriščiūnienė, V.; Krikštolaitytė, S.; Šačkus, A. Colorimetric Cyanide Chemosensor Based on 1′,3,3′,4-Tetrahydrospiro[chromene-2,2′-indole]. ChemistryOpen 2015, 4, 363–369. [Google Scholar] [CrossRef]








| Entry | Compound | λmax of 7 (nm) | ε × 103 (dm3·mol−1·cm−1) | Compound | λmax of 8 (nm) | ε × 103 (dm3·mol−1·cm−1) |
|---|---|---|---|---|---|---|
| 1 | 7a | 203 242 385 | 56.5 39.0 29.8 | 8a | 201 247 290 370 544 | 57.2 16.6 13.0 6.2 38.8 |
| 2 | 7b | 205 241 393 | 59.9 19.4 27.2 | 8b | 202 246 291 574 | 57.4 15.4 12.4 43.8 |
| 3 | 7c | 240 383 | 16.4 22.2 | 8c | 246 296 546 | 14.3 10.2 31.6 |
| 4 | 7d | 204 240 316 394 | 49.4 16.4 8.8 18.4 | 8d | 203 245 300 579 | 50.0 14.0 10.6 34.0 |
| 5 | 7e | 214 247 291 298 382 | 39.8 60.8 13.6 13.0 23.0 | 8e | 206 249 290 298 357 541 | 40.2 56.2 56.6 15.6 9.0 34.4 |
| 6 | 7f | 205 247 292 307 392 | 39.6 51.2 14.0 11.4 21.8 | 8f | 208 249 290 300 351 573 | 41.4 45.2 1.2 13.0 5.6 36.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagilienė, M.; Markuckaitė, G.; Krikštolaitytė, S.; Šačkus, A.; Martynaitis, V. Cyanide Anion Determination Based on Nucleophilic Addition to 6-[(E)-(4-Nitrophenyl)diazenyl]-1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indole] Derivatives. Chemosensors 2022, 10, 185. https://doi.org/10.3390/chemosensors10050185
Dagilienė M, Markuckaitė G, Krikštolaitytė S, Šačkus A, Martynaitis V. Cyanide Anion Determination Based on Nucleophilic Addition to 6-[(E)-(4-Nitrophenyl)diazenyl]-1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indole] Derivatives. Chemosensors. 2022; 10(5):185. https://doi.org/10.3390/chemosensors10050185
Chicago/Turabian StyleDagilienė, Miglė, Giedrė Markuckaitė, Sonata Krikštolaitytė, Algirdas Šačkus, and Vytas Martynaitis. 2022. "Cyanide Anion Determination Based on Nucleophilic Addition to 6-[(E)-(4-Nitrophenyl)diazenyl]-1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indole] Derivatives" Chemosensors 10, no. 5: 185. https://doi.org/10.3390/chemosensors10050185
APA StyleDagilienė, M., Markuckaitė, G., Krikštolaitytė, S., Šačkus, A., & Martynaitis, V. (2022). Cyanide Anion Determination Based on Nucleophilic Addition to 6-[(E)-(4-Nitrophenyl)diazenyl]-1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indole] Derivatives. Chemosensors, 10(5), 185. https://doi.org/10.3390/chemosensors10050185