Investigation of the Atomic Layer Deposition of the Titanium Dioxide (TiO2) Film as pH Sensor Using a Switched Capacitor Amplifier
Abstract
:1. Introduction
2. Materials and Methods
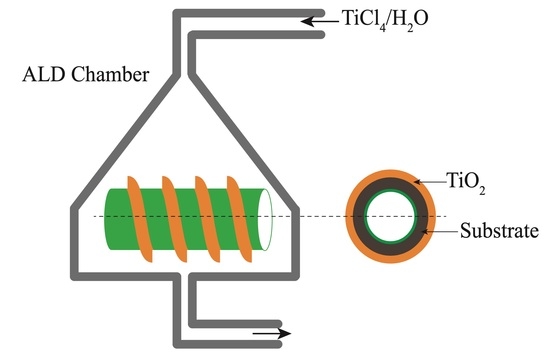
2.1. Sample Preparation
2.2. Electrical Model of Electrode–Electrolyte–Sample Interface
2.3. Measurement Setup
3. Results and Discussion
3.1. Capacitance Measurement
3.2. Series Resistance Measurement
3.3. Drift Measurement
3.4. The pH Response Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALD | Atomic Layer Deposition |
| ISFET | Ion-Sensitive Field Effect Transistors |
| RE | Reference Electrode |
| CE | Counter Electrode |
| LPF | Low Pass Filter |
| HPF | High Pass Filter |
References
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuat. Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.H.; Chou, J.C. Preparation and characterization of the titanium dioxide thin films used for pH electrode and procaine drug sensor by sol–gel method. Mater. Chem. Phys. 2009, 114, 542–548. [Google Scholar] [CrossRef]
- Yin, L.T.; Chou, J.C.; Chung, W.Y.; Sun, T.P.; Hsiung, S.K. Characteristics of silicon nitride after O2 plasma surface treatment for pH-ISFET applications. IEEE Trans. Biomed. Eng. 2001, 48, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Jakobson, C.; Dinnar, U.; Feinsod, M.; Nemirovsky, Y. Ion-sensitive field-effect transistors in standard CMOS fabricated by post processing. IEEE Sens. J. 2002, 2, 279–287. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst Limit in Dual-Gated Si-Nanowire FET Sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef]
- Bahari, N.; Zain, A.M.; Abdullah, A.Z.; Sheng, D.B.C.; Othman, M. Study on pH sensing properties of RF magnetron sputtered tantalum pentoxide (Ta2O5) thin film. In Proceedings of the 2010 IEEE International Conference on Semiconductor Electronics (ICSE2010), Malacca, Malaysia, 28–30 June 2010; pp. 76–78. [Google Scholar] [CrossRef]
- Chin, Y.L.; Chou, J.C.; Sun, T.P.; Liao, H.K.; Chung, W.Y.; Hsiung, S.K. A novel SnO2/Al discrete gate ISFET pH sensor with CMOS standard process. Sens. Actuat. B Chem. 2001, 75, 36–42. [Google Scholar] [CrossRef]
- Fredj, Z.; Baraket, A.; Ben Ali, M.; Zine, N.; Zabala, M.; Bausells, J.; Elaissari, A.; Benson, N.U.; Jaffrezic-Renault, N.; Errachid, A. Capacitance Electrochemical pH Sensor Based on Different Hafnium Dioxide (HfO2) Thicknesses. Chemosensors 2021, 9, 13. [Google Scholar] [CrossRef]
- Yao, P.C.; Chiang, J.L.; Lee, M.C. Application of sol–gel TiO2 film for an extended-gate H+ ion-sensitive field-effect transistor. Solid State Sci. 2014, 28, 47–54. [Google Scholar] [CrossRef]
- Liu, J.; de la Garza, L.; Zhang, L.; Dimitrijevic, N.M.; Zuo, X.; Tiede, D.M.; Rajh, T. Photocatalytic probing of DNA sequence by using TiO2/dopamine-DNA triads. Chem. Phys. 2007, 339, 154–163. [Google Scholar] [CrossRef]
- Liu, H.; Sun, N.; Ding, P.; Chen, C.; Wu, Z.; Zhu, W.; Liu, L.; Wang, Z.; Pei, R. Fabrication of aptamer modified TiO2 nanofibers for specific capture of circulating tumor cells. Coll. Surf. B Biointerf. 2020, 191, 110985. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Chou, J.C.; Liao, L.P. Study of TiO2Thin Films for Ion Sensitive Field Effect Transistor Application with RF Sputtering Deposition. Jpn. J. Appl. Phys. 2004, 43, 61–65. [Google Scholar] [CrossRef]
- Battiston, G.A.; Gerbasi, R.; Porchia, M.; Marigo, A. Influence of substrate on structural properties of TiO2 thin films obtained via MOCVD. Thin Solid Films 1994, 239, 186–191. [Google Scholar] [CrossRef]
- Avci, N.; Smet, P.F.; Poelman, H.; de Velde, N.V.; Buysser, K.D.; Driessche, I.V.; Poelman, D. Characterization of TiO2 powders and thin films prepared by non-aqueous sol–gel techniques. J. -Sol-Gel Sci. Technol. 2009, 52, 424–431. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Hu, H.; Chen, F.; Kong, M.; Zhang, Q.; Luo, T.; Zhao, L.; Guo, Z.; Li, J.; et al. Atomic Layer Deposition of TiO2 for a High-Efficiency Hole-Blocking Layer in Hole-Conductor-Free Perovskite Solar Cells Processed in Ambient Air. ACS Appl. Mater. Interfaces 2016, 8, 17999–18007. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M.; Nykänen, E.; Soininen, P.; Niinistö, L. Growth of titanium dioxide thin films by atomic layer epitaxy. Thin Solid Films 1993, 225, 288–295. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Physics 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Uustare, T. Atomic layer deposition of titanium dioxide from TiCl4 and H2O: Investigation of growth mechanism. Appl. Surf. Sci. 2001, 172, 148–158. [Google Scholar] [CrossRef]
- Pessoa, R.; Chiappim Junior, W.; Testoni, G.; Lima, J.; Maciel, H.; Vieira, L. Effect of Process Temperature and Reaction Cycle Number on Atomic Layer Deposition of TiO2 Thin Films Using TiCl4 and H2O Precursors: Correlation Between Material Properties and Process Environment. Braz. J. Phys. 2015, 46, 56–59. [Google Scholar]
- Mirtaheri, P.; Grimnes, S.; Martinsen, G. Electrode polarization impedance in weak NaCl aqueous solutions. IEEE Trans. Biomed. Eng. 2005, 52, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- High Performance, 145 MHz FastFET Op Amps; Analog Devices: Norwood, MA, USA, 2016.













| Sample | Capacitance (F) | Series Resistance () | Drift (mV/s) | pH Sensitivity (mV/pH) |
|---|---|---|---|---|
| 20 nm, 300 °C | 14.6 | 568 | 0.077 | 33.7 |
| 40 nm, 300 °C | 7.3 | 549 | 0.125 | 39 |
| 80 nm, 300 °C | 5.5 | 497 | 0.174 | 40 |
| 20 nm, 150 °C | 19.7 | 1061 | 0.165 | 21 |
| 40 nm, 150 °C | 11.7 | 995 | 0.211 | 23.7 |
| 80 nm, 150 °C | 8.1 | 884 | 0.145 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nematzadeh, M.; Nilsen, O.; Häfliger, P.D.; Killi, V.A.-L.K. Investigation of the Atomic Layer Deposition of the Titanium Dioxide (TiO2) Film as pH Sensor Using a Switched Capacitor Amplifier. Chemosensors 2022, 10, 274. https://doi.org/10.3390/chemosensors10070274
Nematzadeh M, Nilsen O, Häfliger PD, Killi VA-LK. Investigation of the Atomic Layer Deposition of the Titanium Dioxide (TiO2) Film as pH Sensor Using a Switched Capacitor Amplifier. Chemosensors. 2022; 10(7):274. https://doi.org/10.3390/chemosensors10070274
Chicago/Turabian StyleNematzadeh, Mozhdeh, Ola Nilsen, Philipp Dominik Häfliger, and Veronica Anne-Line Kathrine Killi. 2022. "Investigation of the Atomic Layer Deposition of the Titanium Dioxide (TiO2) Film as pH Sensor Using a Switched Capacitor Amplifier" Chemosensors 10, no. 7: 274. https://doi.org/10.3390/chemosensors10070274
APA StyleNematzadeh, M., Nilsen, O., Häfliger, P. D., & Killi, V. A. -L. K. (2022). Investigation of the Atomic Layer Deposition of the Titanium Dioxide (TiO2) Film as pH Sensor Using a Switched Capacitor Amplifier. Chemosensors, 10(7), 274. https://doi.org/10.3390/chemosensors10070274