Chitosan Homogenizing Coffee Ring Effect for Soil Available Potassium Determination Using Laser-Induced Breakdown Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation
2.2. Extractant and Chitosan Solution Preparation
2.3. Substrate Selection and Fabrication
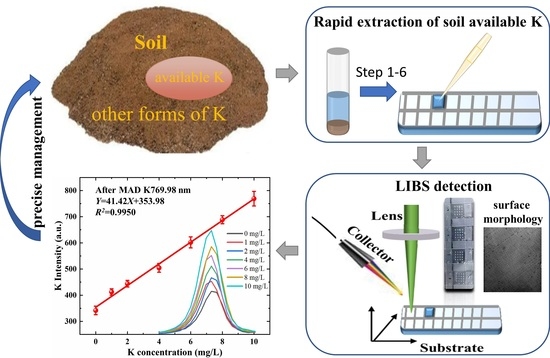
2.4. Samples Preparation
- (1)
- Soil samples (0.6 g) and the extractant of ammonium acetate (6 mL) was mixed in a 10-mL centrifuge tube according to the soil–liquid ratio of 1:10 (the same as CNS method). Then, the mixture was oscillated for a certain time at high frequencies. The optimal combination of frequency and time was optimized based on the relative deviation between our method and the CNS method in this study.
- (2)
- The soil–liquid mixture was centrifuged for three minutes. At this point, there might be some small dead leaves floating in the supernatant.
- (3)
- Diluting the soil solution was performed using a pipette to take about 4 mL of the supernatant into a 50-mL centrifuge tube and recording its mass, m1. The content of dead leaves was different. In order not to inhale dead leaves, the liquid volume taken by the pipette was slightly different. According to the liquid volume and m1, calculating of the density, ρ1, was performed. Then, we added ammonium acetate solution into the centrifuge tube to about 25 mL and recorded the quality, m2, at this point. To calculate the dilution volume ratio, we needed to know the density of the diluted solution. The specific steps were as follows: take out 1 mL of the diluted solution with a pipette first, record the quality difference, and then inject it into the centrifugal tube, calculating the liquid density, ρ2, according to the quality difference of 1 mL of liquid. Finally, according to the mass ratio, m2/m1, and density ratio, ρ2/ρ1, the dilution volume ratio (α) was calculated using the formula α= m2ρ1/m1ρ2.
- (4)
- Adding chitosan solution was performed by taking 0.5 mL of the diluted soil solution into a new centrifugal tube. Then, after adding 1 mL of chitosan solution, the mixture was oscillated for 1 min to ensure the uniformity.
- (5)
- Using a pipette to drop the liquid above on the batch-detection fixed area aluminum substrate, the surface of the substrate was pasted with transparent adhesive tape to form square holes. The liquid was evenly spread over the square hole. The substrate material, square hole area and dropping volume were optimized in this study.
- (6)
- The substrate was dried for 10 min using a heater at 40 °C. At this point, samples were finished.
2.5. Experimental Setup
2.6. SEM/EDS Technology
3. Results and Discussion
3.1. Optimizing the Combination of Oscillation Frequency and Oscillation Time
3.2. Optimizing the Chitosan–Acid Ratio
3.3. Optimizing the Combination of Square Hole Area and Dropping Volume
3.4. SEM/EDS Analysis
3.5. Analysis of Real Samples
3.6. Contrastive Analysis with Other Detection Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holthusen, D.; Peth, S.; Horn, R. Impact of potassium concentration and matric potential on soil stability derived from rheological parameters. Soil Tillage Res. 2010, 111, 75–85. [Google Scholar] [CrossRef]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The effects of potassium fertilization on water-use efficiency in crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Barlog, P.; Grzebisz, W.; Lukowiak, R. Faba bean yield and growth dynamics in response to soil potassium availability and sulfur application. Field Crop. Res. 2018, 219, 87–97. [Google Scholar] [CrossRef]
- Zorb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Prajapati, K. The importance of potassium in plant growth—A review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Chao, X.; Zhang, T.-a.; Lyu, G.; Liang, Z.; Chen, Y. Sustainable application of sodium removal from red mud: Cleaner production of silicon-potassium compound fertilizer. J. Clean Prod. 2022, 352, 131601. [Google Scholar] [CrossRef]
- Borges, R.; Prevot, V.; Forano, C.; Wypych, F. Design and linetic study of sustainable potential slow-release fertilizer obtained by mechanochemical activation of clay minerals and potassium monohydrogen phosphate. Ind. Eng. Chem. Res. 2017, 56, 708–716. [Google Scholar] [CrossRef]
- Fu, X.L.; Zhao, C.J.; Ma, S.X.; Tian, H.W.; Dong, D.M.; Li, G.L. Determining available potassium in soil by laser-induced breakdown spectroscopy combined with cation exchange membrane adsorption. J. Anal. At. Spectrom. 2020, 35, 2697–2703. [Google Scholar] [CrossRef]
- Fu, X.L.; Ma, S.X.; Li, G.L.; Guo, L.B.; Dong, D.M. Rapid detection of chromium in different valence states in soil using resin selective enrichment coupled with laser-induced breakdown spectroscopy: From laboratory test to portable instruments. Spectroc. Acta Pt. B-Atom. Spectr. 2020, 167, 6. [Google Scholar] [CrossRef]
- Kim, H.J.; Hummel, J.W.; Sudduth, K.A.; Motavalli, P.P. Simultaneous analysis of soil macronutrients using ion-selective electrodes. Soil Sci. Soc. Am. J. 2007, 71, 1867–1877. [Google Scholar] [CrossRef]
- Li, T.; Wang, E.; Dong, S.J. G-Quadruplex-based DNAzyme as a sensing platform for ultrasensitive colorimetric potassium detection. Chem. Commun. 2009, 45, 580–582. [Google Scholar] [CrossRef]
- Zhang, L.N.; Zhang, M.; Ren, H.Y.; Pu, P.; Kong, P.; Zhao, H.J. Comparative investigation on soil nitrate-nitrogen and available potassium measurement capability by using solid-state and PVC ISE. Comput. Electron. Agric. 2015, 112, 83–91. [Google Scholar] [CrossRef]
- Liu, F.; Ye, L.H.; Peng, J.Y.; Song, K.L.; Shen, T.T.; Zhang, C.; He, Y. Fast Detection of copper content in rice by laser-induced breakdown spectroscopy with uni- and multivariate analysis. Sensors 2018, 18, 705. [Google Scholar] [CrossRef]
- Peng, J.Y.; Liu, F.; Shen, T.T.; Ye, L.H.; Kong, W.W.; Wang, W.; Liu, X.D.; He, Y. Comparative study of the detection of chromium content in rice leaves by 532 nm and 1064 nm laser-induced breakdown spectroscopy. Sensors 2018, 18, 621. [Google Scholar] [CrossRef]
- De Morais, C.P.; Babos, D.V.; Costa, V.C.; Neris, J.B.; Nicolodelli, G.; Mitsuyuki, M.C.; Mauad, F.F.; Mounier, S.; Milori, D. Direct determination of Cu, Cr, and Ni in river sediments samples using double pulse laser-induced breakdown spectroscopy: Ecological risk and pollution level assessment. Sci. Total Environ. 2022, 837, 155699. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectroc. Acta Pt. B-Atom. Spectr. 2021, 175, 27. [Google Scholar] [CrossRef]
- Li, X.L.; He, Z.N.; Liu, F.; Chen, R.Q. Fast Identification of soybean seed varieties using laser-induced breakdown spectroscopy combined with convolutional neural network. Front. Plant Sci. 2021, 12, 12. [Google Scholar] [CrossRef]
- Noll, R.; Fricke-Begemann, C.; Connemann, S.; Meinhardt, C.; Sturm, V. LIBS analyses for industrial applications—An overview of developments from 2014 to 2018. J. Anal. At. Spectrom. 2018, 33, 945–956. [Google Scholar] [CrossRef]
- Kim, D.; Yang, J.H.; Choi, S.; Yoh, J.J. Analytical methods to distinguish the positive and negative Spectra of mineral and environmental elements using deep ablation laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2018, 72, 896–907. [Google Scholar] [CrossRef]
- Bublitz, J.; Dolle, C.; Schade, W.; Hartmann, A.; Horn, R. Laser-induced breakdown spectroscopy for soil diagnostics. Eur. J. Soil Sci. 2001, 52, 305–312. [Google Scholar] [CrossRef]
- Paris, P.; Piip, K.; Lepp, A.; Lissovski, A.; Aints, M.; Laan, M. Discrimination of moist oil shale and limestone using laser induced breakdown spectroscopy. Spectroc. Acta Pt. B-Atom. Spectr. 2015, 107, 61–66. [Google Scholar] [CrossRef]
- Tian, H.W.; Jiao, L.Z.; Dong, D.M. Rapid determination of trace cadmium in drinking water using laser-induced breakdown spectroscopy coupled with chelating resin enrichment. Sci. Rep. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.A.; Selva, E.J.; Hidalgo, M.; Canals, A. Dispersive liquid-liquid microextraction for metals enrichment: A useful strategy for improving sensitivity of laser-induced breakdown spectroscopy in liquid samples analysis. Talanta 2015, 131, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Zheng, L.J.; Khan, A.Q.; Feng, G.; Zeng, H.P. Laser-induced breakdown spectroscopic detection of trace level heavy metal in solutions on a laser-pretreated metallic target. Talanta 2018, 179, 312–317. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Liu, Y.C.; Pan, J.; Zhang, G.B.; Li, Z.B.; Hu, Z.L.; Chu, Y.W.; Guo, L.B.; Lau, C. Stable and ultrasensitive analysis of organic pollutants and heavy metals by dried droplet method with superhydrophobic-induced enrichment. Anal. Chim. Acta 2021, 1151, 8. [Google Scholar] [CrossRef]
- Wu, M.F.; Wang, X.; Niu, G.H.; Zhao, Z.; Zheng, R.Q.; Liu, Z.; Zhao, Z.J.; Duan, Y.X. Ultrasensitive and simultaneous detection of multielements in aqueous samples based on biomimetic array combined with Laser-induced breakdown spectroscopy. Anal. Chem. 2021, 93, 10196–10203. [Google Scholar] [CrossRef]
- Ahmadi, H.; Jahanshahi, M.; Peyravi, M.; Darzi, G.N. A new antibacterial insight of herbal chitosan-based membranes using thyme and garlic medicinal plant extracts. J. Clean Prod. 2022, 334, 13. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- You, Z.H.; Qiu, Q.M.; Chen, H.Y.; Feng, Y.Y.; Wang, X.; Wang, Y.X.; Ying, Y.B. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 7. [Google Scholar] [CrossRef]
- Aguirre, M.A.; Legnaioli, S.; Almodovar, F.; Hidalgo, M.; Palleschi, V.; Canals, A. Elemental analysis by surface-enhanced Laser-Induced Breakdown Spectroscopy combined with liquid-liquid microextraction. Spectroc. Acta Pt. B-Atom. Spectr. 2013, 79-80, 88–93. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.D.; Lv, Y.Y.; Liu, F.; Peng, J.Y.; Shen, T.L.; Zhao, Y.; Tang, Y.; Luo, S.M. Quantitative analysis of nutrient elements in soil using single and double-pulse laser-induced breakdown spectroscopy. Sensors 2018, 18, 1526. [Google Scholar] [CrossRef]
- Selen, K.ü.E.; Emel, U.L.; Banu, S.; Zeliha, Y.L.; Hakkı, B.S. Mineral content analysis of root canal dentin using laser-induced breakdown spectroscopy. Restor. Dent. Endod. 2018, 43, e11. [Google Scholar] [CrossRef]
- Luan, H.; Gao, Y.N.; LIU, H.; Wang, M.Y.; Guo, S.J.; Cheng, X.L.; Zhang, L.C.; Guo, W.S. The factors of the determination of soil available potassium. J. Green Sci. Technol. 2016, 54, 159–160. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Ma, S.X.; Tang, Y.; Ma, Y.Y.; Chen, F.; Zhang, D.; Dong, D.M.; Wang, Z.Z.; Guo, L.B. Stability and accuracy improvement of elements in water using LIBS with geometric constraint liquid-to-solid conversion. J. Anal. At. Spectrom. 2020, 35, 967–971. [Google Scholar] [CrossRef]
- Zheng, L.J.; Niu, S.; Khan, A.Q.; Yuan, S.; Yu, J.; Zeng, H.P. Comparative study of the matrix effect in Cl analysis with laser-induced breakdown spectroscopy in a pellet or in a dried solution layer on a metallic target. Spectroc. Acta Pt. B-Atom. Spectr. 2016, 118, 66–71. [Google Scholar] [CrossRef]
- Almeida, M.; Segundo, M.A.; Lima, J.; Rangel, A. Potentiometric multi-syringe flow injection system for determination of exchangeable potassium in soils with in-line extraction. Microchem. J. 2006, 83, 75–80. [Google Scholar] [CrossRef]
- Cox, A.E.; Joern, B.C.; Brouder, S.M.; Gao, D. Plant-available potassium assessment with a modified sodium tetraphenylboron method. Soil Sci. Soc. Am. J. 1999, 63, 902–911. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.N.; Zhang, M. Screening of Pretreatment parameters for novel solid-state ISE-based soil extractable potassium detection. In Proceedings of the IEEE 11th International Conference on Electronic Measurement and Instruments (ICEMI), Harbin, China, 16–19 August 2013; pp. 947–953. [Google Scholar]









| Square Hole Area (mm2) | Surface Density (μL/mm2) | |||
|---|---|---|---|---|
| 5 × 5 | 6 × 6 | 7 × 7 | ||
| Dropping volume (μL) | 10.2 | 14.7 | 20.0 | 0.4 |
| 12.8 | 18.4 | 25.0 | 0.5 | |
| 15.3 | 22.0 | 30.0 | 0.6 | |
| Samples | Dilution Volume Ratio (α) | K 769.98 nm Intensity by REMC-LIBS for Three Replications | RSD (%) | Available K Content by REMC-LIBS (mg/kg) | Available K Content by CNS (mg/kg) | Relative Errors (%) | ||
|---|---|---|---|---|---|---|---|---|
| 8 | 5.41 | 461.71 | 461.11 | 458.30 | 0.40 | 138.97 | 130.38 | 6.59 |
| 9 | 5.53 | 536.70 | 540.96 | 546.39 | 0.90 | 250.16 | 255.49 | 2.09 |
| 10 | 5.77 | 651.92 | 622.30 | 661.92 | 3.25 | 405.93 | 412.56 | 1.61 |
| 11 | 16.68 | 574.73 | 556.48 | 536.39 | 3.45 | 812.32 | 839.05 | 3.19 |
| 12 | 16.23 | 549.32 | 607.76 | 608.57 | 5.77 | 918.62 | 879.90 | 4.40 |
| Method | Soil (g) | Accuracy | Test Time | LOD (ppm) | LOQ 1 (ppm) | Ref. |
|---|---|---|---|---|---|---|
| Potentiometric multi-syringe flow injection system | 5 | RSD < 3.0% | >2 h | 6 | 19.8 | [39] |
| Modified NaBPh4 method | 0.5 | SD 2 (mg/kg) 10–27 | >2 h | <19 | / | [40] |
| All-solid-state K ISE and Ion-selective electrode | 2.5 | RSD 0.2–11.8% | >1 h | 4.8 | 15.8 | [41] |
| CEMA-LIBS | 10 | RE 3 1.36–5.15% | >24 h | 2.2 | 7.3 | [8] |
| REMC-LIBS | 0.6 | RE 1.61–6.59% | <20 min | 0.25 | 0.8 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, R.; You, Z.; Pan, T.; Yang, R.; Huang, J.; Fang, H.; Kong, W.; Peng, J.; Liu, F. Chitosan Homogenizing Coffee Ring Effect for Soil Available Potassium Determination Using Laser-Induced Breakdown Spectroscopy. Chemosensors 2022, 10, 374. https://doi.org/10.3390/chemosensors10090374
Li X, Chen R, You Z, Pan T, Yang R, Huang J, Fang H, Kong W, Peng J, Liu F. Chitosan Homogenizing Coffee Ring Effect for Soil Available Potassium Determination Using Laser-Induced Breakdown Spectroscopy. Chemosensors. 2022; 10(9):374. https://doi.org/10.3390/chemosensors10090374
Chicago/Turabian StyleLi, Xiaolong, Rongqin Chen, Zhengkai You, Tiantian Pan, Rui Yang, Jing Huang, Hui Fang, Wenwen Kong, Jiyu Peng, and Fei Liu. 2022. "Chitosan Homogenizing Coffee Ring Effect for Soil Available Potassium Determination Using Laser-Induced Breakdown Spectroscopy" Chemosensors 10, no. 9: 374. https://doi.org/10.3390/chemosensors10090374
APA StyleLi, X., Chen, R., You, Z., Pan, T., Yang, R., Huang, J., Fang, H., Kong, W., Peng, J., & Liu, F. (2022). Chitosan Homogenizing Coffee Ring Effect for Soil Available Potassium Determination Using Laser-Induced Breakdown Spectroscopy. Chemosensors, 10(9), 374. https://doi.org/10.3390/chemosensors10090374