Synthesis and Modification of Magnetic Nanoparticles for Biosensing and Bioassay Applications: A Review
Abstract
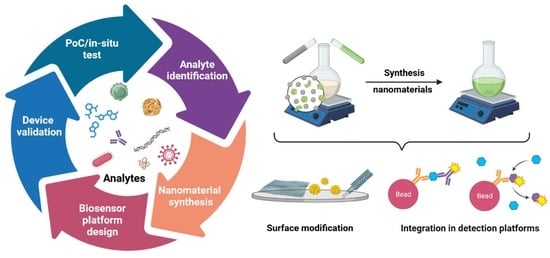
:1. Introduction
- Magnetic properties: Due to their inherent magnetism, MNPs are easily manipulated and controlled by external magnetic fields, which is advantageous in biomedical applications, such as drug delivery and hyperthermia, where localization and targeted delivery are critical.
- Biocompatibility: Many MNPs, especially those coated with biocompatible materials, such as polyethylene glycol (PEG), surfactants, and proteins, exhibit low toxicity, making them suitable for biomedical applications.
- Drug delivery: MNPs can be functionalized with biomolecules or specific ligands to carry drugs or therapeutic agents. Magnetic guidance of these nanoparticles can improve the efficiency and precision of drug delivery, improving therapeutic outcomes and minimizing side effects.
- Biomedical imaging and diagnostics: MNPs have exceptional capabilities for high-resolution imaging and early disease detection. In this regard, MNPs serve as contrast agents in various imaging modalities such as magnetic resonance imaging (MRI), magnetic particle imaging (MPI), magnetic particle spectroscopy (MPS), multimodal PET-MRI, SPECT-MRI, and OI-MRI.
- Magnetic hyperthermia: MNPs exposed to alternating magnetic fields can generate heat due to hysteresis losses. This property can be used in magnetic hyperthermia treatments for cancer, where targeted heating of tumor cells can destroy them without affecting healthy tissue.
- Environmental applications: MNPs can be used as sorbents in solid–liquid extraction. Due to their magnetic nature, they can be used for separation, treatment, and remediation processes to remove pollutants and contaminants from water and soil.
- Nanotechnology integration: MNPs can be easily integrated into existing nanotechnology platforms, allowing them to be used together with other nanomaterials to create hybrid systems with enhanced functionalities, including hybrid analytical methods such as magnetic bioassays and biosensors.
- Catalysis: MNPs can serve as effective catalysts in various chemical reactions due to their large surface area and unique magnetic properties. They can be easily separated and reused, which makes them attractive for catalytic applications.
- Biocompatibility: Some MNPs may cause adverse reactions or toxicity in biological systems or suffer denaturation of the bioreceptor during the test. Because of this effect, the safe use of MNPs in the human body requires appropriate surface modification/coating and biocompatibility and toxicological testing prior to commercialization.
- Aggregation: MNPs can aggregate or cluster together, which affects their stability and uniformity, modifying their interaction with biological tissues and leading to unpredictable behaviors, thereby reducing their efficacy. In addition, these phenomena may decrease analytical performance in bioassays, hindering electron transfer reactions or decreasing the number of active sites. Again, surface functionalization with adequate modifiers (silanizing agents, polymers, grafting specific functional groups) or the use of stabilizing solution, when possible, can improve stability and prevent aggregation.
- Sensitivity and signal-to-noise ratio: Integration of MNPs may affect the test sensitivity and limit their effectiveness in detection systems. In recent years, many different approaches have been proposed to overcome these difficulties, such as the incorporation of (bio)catalytic labels, enzymatic amplifications, proximity ligation assays, nucleic acid-based amplification strategies such as PCR, hybridization chain reaction, rolling circle amplification, etc.
- Interference with biological molecules: MNPs could interact/interfere with biological molecules, reducing their effectiveness and analytical performance. To overcome these difficulties, careful design of the MNP surface, its coating, and modifications are needed to minimize interference with biological molecules while maintaining their functionality.
2. Synthesis and Modification of MNPs
2.1. Synthesis of MNPs
- Vapor Condensation: In this technique, vaporized metal atoms or compounds are rapidly cooled to form nanoparticles through condensation. The size of nanoparticles depends on the temperature, pressure, and cooling rate.
- Laser Ablation: High-energy laser pulses are used to vaporize a target material, and the ejected material condenses to form nanoparticles. This method allows the synthesis of nanoparticles without the need for chemical reagents.
- Sputtering: In this process, energetic ions are bombarded onto a solid target material, causing the ejection of atoms and their deposition on a substrate, resulting in the formation of nanoparticles.
- Ball Milling: This mechanical method consists of grinding and mixing solid materials, which results in the formation of nanoparticles due to high-energy collisions between the particles.
- Co-precipitation or chemical reduction: In this method, metal ions are dissolved in a solution and reduced to form nanoparticles by the addition of a reducing agent. The size and shape of the nanoparticles can be controlled by adjusting the reaction conditions and stabilizing agents.
- Solvothermal method: This technique involves the hydrolysis and condensation of metal alkoxides or metal chlorides in a solution to produce a colloidal suspension of nanoparticles. The process allows precise control of nanoparticle composition and size.
- Thermal decomposition: In this procedure, high temperatures are used to decompose precursors and produce nuclei, followed by their subsequent growth into NPs. Several factors such as temperature, solvent, reactant ratio, reflux time, and seed concentration are important to determine the size and morphology of nanoparticles.
- Micro-emulsion: Nanoparticles are formed into a stable microemulsion, where the core contains the reaction precursors and the surfactants control the particle size and prevent aggregation.
- Green synthesis: This approach uses plant extracts or other natural sources as reducing and stabilizing agents to produce nanoparticles. It offers an eco-friendly alternative to conventional chemical methods.
2.1.1. Co-Precipitation
2.1.2. Solvothermal Synthesis
2.1.3. Thermal Decomposition
2.1.4. Microemulsion Method
2.1.5. Green Synthesis
| Sensor Material | Synthesis Method | Analyte | LoD (nM) | Linear Range | Reference |
|---|---|---|---|---|---|
| ZnxFe3−xO4 | co-precipitation | glucose | 0.03 mM | 0.1 to 2 mM | [54] |
| MnFe2O4@chitosan/MWCNTs/PDMS | co-precipitation | alpha2-macroglobulin | 0.13 ng/mL | 10 ng/mL to 100 μg/mL | [56] |
| Cerium-doped magnetite nanoparticles | co-precipitation | metronidazole | 0.391 mol/L | 3.47 to 93.7 μmol/L | [57] |
| Fe3O4@Cu@Cu2O | solvothermal | H2O2 | 0.2 mM | 0.4 to 1.5 mM | [58] |
| Au@Fe3O4 | solvothermal | ochratoxin A | 30 pg/mL | 0.5–100 ng/mL | [59] |
| Fe3O4@ZIF-8/RGO | solvothermal | dopamine | 0.667 nM | 2.0 nM to 10 μM | [60] |
| Fe3O4@CTAB | thermal decomposition | H2O2 | 103 μmol/L | 100 μmol/L to 1.8 mmol/L | [61] |
| Iron oxide MNPs | green synthesis | mercury | 0.004 ppm | 0.030 to 0.060 ppm | [67] |
| silica/Fe3O4@C@Ag | green synthesis | cholesterol | 0.5 mM | 0.5 to 22.5 mM | [68] |
2.2. Surface Modifications of MNPs
2.2.1. Organic Coatings/Ligands
Chitosan
Poly-(Ethylene Glycol)
Polypyrrole (Ppy)
Polyaniline (PANI)
Polydopamine (pDA)
2.2.2. Inorganic Coatings and Modifications
Silica
Gold (Au)
Platinum (Pt)
Quantum Dots (QDs)
| Sensor Material | MNPs Surface Modification/ Coatings | Analyte | LoD | Linear Range | Reference |
|---|---|---|---|---|---|
| MNP/CG | chitosan | glucose | 16 μM | Up to 26 mM | [76] |
| Fe3O4-chitosan-β-cyclodextrin/MWCNTs | chitosan | glucose | 19.30 μM | 40 μM to 1.04 mM | [77] |
| CNP-L/CuONP/MWCNT/Pe/GC | chitosan | triglycerides | 3.2 mg/L | 9.6 to 11 mg/L | [78] |
| Fe3O4@Au@PEG@HA | polyethylene glycol | brucellosis antibodies | 0.36 fg/mL | 10 fg/mL to 10 pg/mL | [85] |
| PEG-MNPs | poly ethylene glycol | glucose | 3 μM | 5 to 1000 μM | [86] |
| APBA-PEG-MNs | polyethylene glycol | Staphylococcus aureus | 270 CFU/mL | 100 to 100,000 CFU/mL | [87] |
| PPy and PPy-containing CS/Fe3O4 | polypyrrole | glucose-6-phosphate | 0.002 mM | 0.0025 to 0.05 mM | [89] |
| Ppy NPs | polypyrrole | C-reactive protein | 0.45 mg/L | 0.75 to 12 mg/L | [90] |
| Fe3O4@PANI | polyaniline | catechol | 0.2 nM | - | [91] |
| Fe3O4@PANI NPs | polyaniline | creatinine | 0.35 nM | 0.02 to 1 μM | [92] |
| PANI/MG | polyaniline | hydroquinone | 2.94 μM | 0.4 to 337.2 μM | [93] |
| GOx-Au-pDA-Fe3O4 MBNPs | polydopamine | glucose | 6.5 mM | 0.02 to 1.87 mM | [95] |
| MNPs@pDA-Ab | polydopamine | Legionella pneumophila | 10,000 CFU/mL | 10 to 100,000 CFU/mL | [96] |
| MNPs@pDA | polydopamine | H2O2 | 0.23 μM | 0.5 to 30 μM | [97] |
| MNP-MSN@CUR@ZnO@pAbs | silica | Salmonella Typhimurium | Colorimetric: 63 CFU/mL Fluorescent: 40 CFU/mL | 102 to 107 CFU/mL | [99] |
| Silica-coated MNPs | silica | dopamine, uric acid and folic acid | 12, 14 and 18 nM | 1 to 30.6, 1 to 286 and 1 to 369 μM | [100] |
| 3D mag-MoO3–PDA@Au NS | gold | SARS-CoV-2 | 10 fg/mL | 10 fg/mL to 1 ng/mL | [101] |
| gold-coated magnetic nanoparticles | gold | leukemia cells | 10 cells/mL | 10 to 1,000,000 cells/mL | [103] |
| DTSSP-AuNPs | gold | dopamine | 10 nM | 0.02 to 0.80 μM | [104] |
| MrGO@AuNPs | gold | bisphenol A | 0.141 pg/mL | 0.01 ng/mL to 100 ng/mL | [105] |
| AuNPs/BSA/Fe3O4 | gold | glucose | 3.54 μM | 0.25 to 7.0 mM | [106] |
| Fe3O4@Au NPs | gold | Pb2+ | 15 pM | 50 pM to 1 μM | [108] |
| Magnetic beads and Pt NPs | platinum | thyroid stimulant hormone | 0.004 mU/L | 0.013 to 12 mU/L | [109] |
| MPt/CS NPs | platinum | Human chorionic gonadotropin | 0.039 ng/mL | - | [110] |
| PtNP-PAMAM-MNP/GO-CMCw | platinum | Xanthine | 13 nM | 50 nM to 12 μM | [111] |
| SPIONs and CdTe-MPA QDsx | QD | Escherichia coli | 100 bacterial cells | 100 to 400 μg/mL | [113] |
| NAC-CQDsy | QD | histamine | 21.15 ppb | 0.1 to 100 ppm | [114] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for chemists of terms used in biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Choi, J.R. Development of Point-of-Care Biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef]
- Pioz, M.J.; Espinosa, R.L.; Laguna, M.F.; Santamaria, B.; Murillo, A.M.M.; Hueros, Á.L.; Quintero, S.; Tramarin, L.; Valle, L.G.; Herreros, P.; et al. A review of Optical Point-of-Care devices to Estimate the Technology Transfer of These Cutting-Edge Technologies. Biosensors 2022, 12, 1091. [Google Scholar] [CrossRef]
- Qazi, S.; Raza, K. Chapter 4—Smart biosensors for an efficient point of care (PoC) health management. In Smart Biosensors in Medical Care; Chaki, J., Dey, N., De, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 65–85. [Google Scholar]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Calabrese, A.; Battistoni, P.; Ceylan, S.; Zeni, L.; Capo, A.; Varriale, A.; D’Auria, S.; Staiano, M. An Impedimetric Biosensor for Detection of Volatile Organic Compounds in Food. Biosensors 2023, 13, 341. [Google Scholar] [CrossRef]
- Hurot, C.; Brenet, S.; Buhot, A.; Barou, E.; Belloir, C.; Briand, L.; Hou, Y. Highly sensitive olfactory biosensors for the detection of volatile organic compounds by surface plasmon resonance imaging. Biosens. Bioelectron. 2019, 123, 230–236. [Google Scholar] [CrossRef]
- Cheon, J.; Qin, J.; Lee, L.P.; Lee, H. Advances in Biosensor Technologies for Infection Diagnostics. Acc. Chem. Res. 2022, 55, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-San Martín, C.; Montero-Calle, A.; Garranzo-Asensio, M.; Gamella, M.; Pérez-Ginés, V.; Pedrero, M.; Pingarrón, J.M.; Barderas, R.; de-Los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; et al. First bioelectronic immunoplatform for quantitative secretomic analysis of total and metastasis-driven glycosylated haptoglobin. Anal. Bioanal. Chem. 2023, 415, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.; O’Reilly, E.L.; McComb, C.; Macrae, A.I.; Eckersall, P.D. An immunoturbidimetric assay for bovine haptoglobin. Comp. Clin. Pathol. 2019, 28, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.; Fan, L.; Jiang, T.; Zhang, Y. Advances in nanoparticle-based lateral flow immunoassay for point-of-care testing. View 2022, 3, 20200125. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. ChemMedChem 2022, 17, e202200142. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Zhong, Z.; He, J.; Li, G.; Xia, L. Recent Advances in Magnetic Nanoparticles-Assisted Microfluidic Bioanalysis. Chemosensors 2023, 11, 173. [Google Scholar] [CrossRef]
- Kumar, N.S.; Suvarna, R.P.; Naidu, K.C.B.; Reddy, B.V.S. 19—Magnetic nanoparticles for high energy storage applications. In Fundamentals and Industrial Applications of Magnetic Nanoparticles; Hussain, C.M., Patankar, K.K., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 601–618. [Google Scholar]
- Salazar-Carballo, P.A.; López-Pérez, M.; Martín-González, M.E.; Hernández-Suarez, F.; Martín-Luis, M.C. Radon Dynamics and Effective Dose Estimation in a Touristic Volcanic Cave: La Cueva del Viento, Tenerife (Canary Islands, Spain). GeoHealth 2023, 7, e2022GH000704. [Google Scholar] [CrossRef]
- Carinelli, S.; Fernández, I.; González-Mora, J.L.; Salazar-Carballo, P.A. Hemoglobin-modified nanoparticles for electrochemical determination of haptoglobin: Application in bovine mastitis diagnosis. Microchem. J. 2022, 179, 107528. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Rabiei, H.; Tahmasbi, B.; Ghasemi, B.; Mardi, F. Preparation of DSA@MNPs and application as heterogeneous and recyclable nanocatalyst for oxidation of sulfides and oxidative coupling of thiols. Res. Chem. Intermed. 2016, 42, 5723–5737. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Wang, D.; Zhao, L.; Qiao, X.; Zhang, X.; Liu, W.; Wang, C.; Zhou, J. Genetically magnetic control of neural system via TRPV4 activation with magnetic nanoparticles. Nano Today 2021, 39, 101187. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.K.; Ramchandani, M.; Basak, T. Recent Advancements, Challenges, and Future Prospects in Usage of Nanoformulation as Theranostics in Inflammatory Diseases. J. Nanotheranostics 2023, 4, 106–126. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Conde-Díaz, A.; Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Salazar-Carballo, P.Á.; Rodríguez-Delgado, M.Á. Application of polyaniline-based magnetic-dispersive-solid-phase microextraction combined with liquid chromatography tandem mass spectrometry for the evaluation of plastic migrants in food matrices. J. Chromatogr. A 2022, 1670, 462988. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Magnetic Bead-Based Electrochemical Immunoassays On-Drop and On-Chip for Procalcitonin Determination: Disposable Tools for Clinical Sepsis Diagnosis. Biosensors 2020, 10, 66. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fraga-García, P.; Blank-Shim, S.A.; Straub, T.; Haslbeck, M.; Muraca, F.; Dawson, K.A.; Berensmeier, S. Magnetic One-Step Purification of His-Tagged Protein by Bare Iron Oxide Nanoparticles. ACS Omega 2019, 4, 3790–3799. [Google Scholar] [CrossRef]
- Peuker, U.A.; Thomas, O.; Hobley, T.J.; Franzreb, M.; Berensmeier, S.; SchÄFer, M.; Hickstein, B. Bioseparation, Magnetic Particle Adsorbents. Encycl. Ind. Biotechnol. 2010, 1–31. [Google Scholar] [CrossRef]
- Phouthavong, V.; Yan, R.; Nijpanich, S.; Hagio, T.; Ichino, R.; Kong, L.; Li, L. Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods. Materials 2022, 15, 1053. [Google Scholar] [CrossRef]
- Maharana, M.; Sen, S. Magnetic zeolite: A green reusable adsorbent in wastewater treatment. Mater. Today Proc. 2021, 47, 1490–1495. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Santana-Mayor, Á.; Salazar-Carballo, P.Á.; Rodríguez-Delgado, M.Á. Sustainable polypyrrole-based magnetic-microextraction of phthalates from jellies and apple-based beverages prior to tandem mass spectrometry analysis. J. Chromatogr. A 2021, 1637, 461858. [Google Scholar] [CrossRef]
- Villafañe, G.; Bazán, V.; Brandaleze, E.; López, A.; Pacheco, P.; Maratta, A. Solid phase extraction of arsenic on modified MWCNT/Fe3O4 magnetic hybrid nanoparticles from copper ores samples with ETAAS determination. Talanta Open 2022, 6, 100149. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-Ordered Mesoporous Silica Films for Electrochemical Detection of Hg(II) Ion in Pharmaceuticals and Soil Samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef]
- Abdulla, M.; Ali, A.; Jamal, R.; Bakri, T.; Wu, W.; Abdiryim, T. Electrochemical Sensor of Double-Thiol Linked PProDOT@Si Composite for Simultaneous Detection of Cd(II), Pb(II), and Hg(II). Polymers 2019, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Scigalski, P.; Kosobucki, P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules 2020, 25, 4869. [Google Scholar] [CrossRef]
- Fernández, I.; Carinelli, S.; González-Mora, J.L.; Villalonga, R.; Lecuona, M.; Salazar-Carballo, P.A. Electrochemical bioassay based on l-lysine-modified magnetic nanoparticles for Escherichia coli detection: Descriptive results and comparison with other commercial magnetic beads. Food Control 2023, 145, 109492. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Gamella, M.; Serafin, V.; Yanez-Sedeno, P.; Pingarron, J.M. Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices. Sensors 2020, 20, 3376. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Pedrero, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensors for autoantibodies in autoimmune and cancer diseases. Anal. Methods 2019, 11, 871–887. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Miandoab, M.P.; Şaşmaz, M. Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review. Micromachines 2021, 12, 768. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Cui, G.; Wu, X.; Yang, M.; Piao, Y.; Bai, Z.; Wang, L.; Kraft, M.; Zhao, W.; et al. A magnetic nanoparticle assisted microfluidic system for low abundance cell sorting with high recovery. Micro Nano Eng. 2022, 15, 100136. [Google Scholar] [CrossRef]
- Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Serafin, V.; Yanez-Sedeno, P.; Pingarron, J.M. Cutting-Edge Advances in Electrochemical Affinity Biosensing at Different Molecular Level of Emerging Food Allergens and Adulterants. Biosensors 2020, 10, 10. [Google Scholar] [CrossRef]
- Mohsin, A.; Hussain, M.H.; Mohsin, M.Z.; Zaman, W.Q.; Aslam, M.S.; Shan, A.; Dai, Y.; Khan, I.M.; Niazi, S.; Zhuang, Y.; et al. Recent Advances of Magnetic Nanomaterials for Bioimaging, Drug Delivery, and Cell Therapy. ACS Appl. Nano Mater. 2022, 5, 10118–10136. [Google Scholar] [CrossRef]
- Ma, K.; Xu, S.; Tao, T.; Qian, J.; Cui, Q.; Rehman, S.U.; Zhu, X.; Chen, R.; Zhao, H.; Wang, C.; et al. Magnetosome-inspired synthesis of soft ferrimagnetic nanoparticles for magnetic tumor targeting. Proc. Natl. Acad. Sci. USA 2022, 119, e2211228119. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Tripathy, A.; Nine, M.J.; Silva, F.S. Biosensing platform on ferrite magnetic nanoparticles: Synthesis, functionalization, mechanism and applications. Adv. Colloid Interface Sci. 2021, 290, 102380. [Google Scholar] [CrossRef] [PubMed]
- Schladt, T.D.; Schneider, K.; Schild, H.; Tremel, W. Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalton Trans. 2011, 40, 6315–6343. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging Application of Magnetic Nanoparticles for Diagnosis and Treatment of Cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nunez, J.; Arcentales-Vera, B.; Chichande-Proano, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Ognjanović, M.; Stanković, D.M.; Ming, Y.; Zhang, H.; Jančar, B.; Dojčinović, B.; Prijović, Ž.; Antić, B. Bifunctional (Zn,Fe)3O4 nanoparticles: Tuning their efficiency for potential application in reagentless glucose biosensors and magnetic hyperthermia. J. Alloys Compd. 2019, 777, 454–462. [Google Scholar] [CrossRef]
- Antarnusa, G.; Suharyadi, E. A synthesis of polyethylene glycol (PEG)-coated magnetite Fe3O4 nanoparticles and their characteristics for enhancement of biosensor. Mater. Res. Express 2020, 7, 056103. [Google Scholar] [CrossRef]
- Guo, J.H.X.; Ge, Y.; Zhao, D.; Sang, S.; Ji, J. Highly Sensitive Magnetoelastic Biosensor for Alpha2-Macroglobulin Detection Based on MnFe2O4@chitosan. Micromachines 2023, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Irani-Nezhad, M.H.; Hassandoost, R.; Khataee, A.; Pouran, S.R.; Joo, S.W. Cerium doped magnetite nanoparticles for highly sensitive detection of metronidazole via chemiluminescence assay. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 234, 118272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, M.; Shu, J.; Li, Y. One-step solvothermal synthesis of Fe3O4@Cu@Cu2O nanocomposite as magnetically recyclable mimetic peroxidase. J. Alloys Compd. 2016, 682, 432–440. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; Wang, K.; Yang, X.; Liu, Q.; Hao, N.; Wang, C.; Dong, X.; Huang, X. Colorimetric aptasensing of ochratoxin A using Au@ Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens. Bioelectron. 2016, 77, 1183–1191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Magnetic Fe3O4@MOFs decorated graphene nanocomposites as novel electrochemical sensor for ultrasensitive detection of dopamine. RSC Adv. 2015, 5, 98260–98268. [Google Scholar] [CrossRef]
- Guivar, J.A.; Fernandes, E.G.; Zucolotto, V. A peroxidase biomimetic system based on Fe3O4 nanoparticles in non-enzymatic sensors. Talanta 2015, 141, 307–314. [Google Scholar] [CrossRef]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Jaras, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and functionalization study of microemulsion-prepared magnetic iron oxide nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef]
- Rivas, J.; Redondo, Y.P.; Iglesias-Silva, E.; Vilas-Vilela, J.M.; León, L.M.; López-Quintela, M.A. Air-stable Fe@Au nanoparticles synthesized by the microemulsion’s methods. J. Korean Phys. Soc. 2013, 62, 1376–1381. [Google Scholar] [CrossRef]
- Souza, C.G.S.d.; Souza, J.B.; Beck, W.; Varanda, L.C. Luminomagnetic Silica-Coated Heterodimers of Core/Shell FePt/Fe3O4 and CdSe Quantum Dots as Potential Biomedical Sensor. J. Nanomater. 2017, 2017, 2160278. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Perez-Beltran, C.H.; Garcia-Guzman, J.J.; Ferreira, B.; Estevez-Hernandez, O.; Lopez-Iglesias, D.; Cubillana-Aguilera, L.; Link, W.; Stanica, N.; da Costa, A.M.R.; Palacios-Santander, J.M. One-minute and green synthesis of magnetic iron oxide nanoparticles assisted by design of experiments and high energy ultrasound: Application to biosensing and immunoprecipitation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112023. [Google Scholar] [CrossRef]
- Satvekar, R.K.; Pawar, S.H. Multienzymatic Cholesterol Nanobiosensor Using Core–Shell Nanoparticles Incorporated Silica Nanocomposite. J. Med. Biol. Eng. 2018, 38, 735–743. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal green synthesis of magnetic Fe(3)O(4)-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.u.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Li, Y.; Lee, D.S. Functionalization of Magnetic Nanoparticles with Organic Ligands toward Biomedical Applications. Adv. NanoBiomed Res. 2021, 1, 2000043. [Google Scholar] [CrossRef]
- García, R.S.; Stafford, S.; Gun’ko, Y.K. Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications. Appl. Sci. 2018, 8, 172. [Google Scholar] [CrossRef]
- Kulikova, T.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical DNA Sensors with Layered Polyaniline-DNA Coating for Detection of Specific DNA Interactions. Sensors 2019, 19, 469. [Google Scholar] [CrossRef]
- Gong, Q.; Han, H.; Yang, H.; Zhang, M.; Sun, X.; Liang, Y.; Liu, Z.; Zhang, W.; Qiao, J. Sensitive electrochemical DNA sensor for the detection of HIV based on a polyaniline/graphene nanocomposite. J. Mater. 2019, 5, 313–319. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials 2023, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Zou, R.; Wu, H.; Shi, H.; Yu, S.; Liu, Y. Multifunctional glucose biosensors from Fe(3)O(4) nanoparticles modified chitosan/graphene nanocomposites. Sci. Rep. 2015, 5, 11129. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Luo, Y.; Xiong, H.; Yao, S.; Zhu, M.; Song, H. A Novel Amperometric Glucose Biosensor Based on Fe3O4-Chitosan-β-Cyclodextrin/MWCNTs Nanobiocomposite. Electroanalysis 2020, 33, 723–732. [Google Scholar] [CrossRef]
- Di Tocco, A.; Robledo, S.N.; Osuna, Y.; Sandoval-Cortez, J.; Granero, A.M.; Vettorazzi, N.R.; Martinez, J.L.; Segura, E.P.; Ilina, A.; Zon, M.A.; et al. Development of an electrochemical biosensor for the determination of triglycerides in serum samples based on a lipase/magnetite-chitosan/copper oxide nanoparticles/multiwalled carbon nanotubes/pectin composite. Talanta 2018, 190, 30–37. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Ma, Z.; Wu, B. Effects of pharmaceutical PEGylation on drug metabolism and its clinical concerns. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1691–1702. [Google Scholar] [CrossRef]
- Patsula, V.; Horák, D.; Kučka, J.; Macková, H.; Lobaz, V.; Francová, P.; Herynek, V.; Heizer, T.; Páral, P.; Šefc, L. Synthesis and modification of uniform PEG-neridronate-modified magnetic nanoparticles determines prolonged blood circulation and biodistribution in a mouse preclinical model. Sci. Rep. 2019, 9, 10765. [Google Scholar] [CrossRef]
- Zomorodian, K.; Veisi, H.; Mousavi, S.M.; Ataabadi, M.S.; Yazdanpanah, S.; Bagheri, J.; Mehr, A.P.; Hemmati, S.; Veisi, H. Modified magnetic nanoparticles by PEG-400-immobilized Ag nanoparticles (Fe3O4@PEG–Ag) as a core/shell nanocomposite and evaluation of its antimicrobial activity. Int. J. Nanomed. 2018, 13, 3965–3973. [Google Scholar] [CrossRef]
- Nagasaki, Y.; Kobayashi, H.; Katsuyama, Y.; Jomura, T.; Sakura, T. Enhanced immunoresponse of antibody/mixed-PEG co-immobilized surface construction of high-performance immunomagnetic ELISA system. J. Colloid Interface Sci. 2007, 309, 524–530. [Google Scholar] [CrossRef]
- Ahmad, N.; Colak, B.; Zhang, D.-W.; Gibbs, M.J.; Watkinson, M.; Becer, C.R.; Gautrot, J.E.; Krause, S. Peptide Cross-Linked Poly (Ethylene Glycol) Hydrogel Films as Biosensor Coatings for the Detection of Collagenase. Sensors 2019, 19, 1677. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Sheng, J.; Zhao, S.; Liu, M.; Chen, L. The detection of brucellosis antibody in whole serum based on the low-fouling electrochemical immunosensor fabricated with magnetic Fe3O4@ Au@ PEG@ HA nanoparticles. Biosens. Bioelectron. 2018, 117, 138–144. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, B.-G.; Cho, S.; Lee, J.; Na, H.B.; Kim, M.I. Visual determination of hydrogen peroxide and glucose by exploiting the peroxidase-like activity of magnetic nanoparticles functionalized with a poly(ethylene glycol) derivative. Microchim. Acta 2017, 184, 2115–2122. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, X.; Xiao, F.; Bai, X.; Xu, Q.; Xu, H. A novel PEG-mediated boric acid functionalized magnetic nanomaterials based fluorescence biosensor for the detection of Staphylococcus aureus. Microchem. J. 2022, 178, 107379. [Google Scholar] [CrossRef]
- Cha, B.G.; Lee, D.; Kim, T.; Piao, Y.; Kim, J. Iron Oxide@Polypyrrole Core–Shell Nanoparticles as the Platform for Photothermal Agent and Electrochemical Biosensor. J. Nanosci. Nanotechnol. 2016, 16, 6942–6948. [Google Scholar] [CrossRef]
- Sahin, S.; Ozmen, I.; Bastemur, G.Y.; Ozkorucuklu, S.P. Development of Voltammetric Glucose-6-phosphate Biosensors Based on the Immobilization of Glucose-6-phosphate Dehydrogenase on Polypyrrole- and Chitosan-Coated Fe(3)O(4) Nanoparticles/Polypyrrole Nanocomposite Films. Appl. Biochem. Biotechnol. 2019, 188, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Tao, Y.; Shen, H.; Yang, C.; Tian, T.; Yang, L.; Zhu, Z. A polypyrrole-mediated photothermal biosensor with a temperature and pressure dual readout for the detection of protein biomarkers. Analyst 2022, 147, 2671–2677. [Google Scholar] [CrossRef]
- Chandra, S.; Lang, H.; Bahadur, D. Polyaniline-iron oxide nanohybrid film as multi-functional label-free electrochemical and biomagnetic sensor for catechol. Anal. Chim. Acta 2013, 795, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Zhu, W.; Xue, C.; Wu, J.; Han, Q.; Wang, X.; Zhou, X.; Jiang, H. Novel electrochemical sensing platform based on magnetic field-induced self-assembly of Fe3O4@Polyaniline nanoparticles for clinical detection of creatinine. Biosens. Bioelectron. 2014, 56, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Jing, T.; Zhou, J.; Tian, J.; Zheng, Y.; Wang, C.; Zhao, Z.; Lin, J.; Liu, H.; Zhao, C.; et al. Laccase immobilized polyaniline/magnetic graphene composite electrode for detecting hydroquinone. Int. J. Biol. Macromol. 2020, 149, 1130–1138. [Google Scholar] [CrossRef]
- Hsine, Z.; Mlika, R.; Jaffrezic-Renault, N.; Korri-Youssoufi, H. Recent progress in graphene based modified electrodes for electrochemical detection of dopamine. Chemosensors 2022, 10, 249. [Google Scholar] [CrossRef]
- Peng, H.P.; Liang, R.P.; Zhang, L.; Qiu, J.D. Facile preparation of novel core-shell enzyme-Au-polydopamine-Fe(3)O(4) magnetic bionanoparticles for glucosesensor. Biosens. Bioelectron. 2013, 42, 293–299. [Google Scholar] [CrossRef]
- Martin, M.; Salazar, P.; Jimenez, C.; Lecuona, M.; Ramos, M.J.; Ode, J.; Alcoba, J.; Roche, R.; Villalonga, R.; Campuzano, S.; et al. Rapid Legionella pneumophila determination based on a disposable core-shell Fe3O4@poly(dopamine) magnetic nanoparticles immunoplatform. Anal. Chim. Acta 2015, 887, 51–58. [Google Scholar] [CrossRef]
- Sardaremelli, S.; Hasanzadeh, M.; Seidi, F. Enzymatic recognition of hydrogen peroxide (H2O2) in human plasma samples using HRP immobilized on the surface of poly(arginine-toluidine blue)- Fe3O4 nanoparticles modified polydopamine: A novel biosensor. J. Mol. Recognit. 2021, 34, e2928. [Google Scholar] [CrossRef] [PubMed]
- Rashid, Z.; Soleimani, M.; Ghahremanzadeh, R.; Vossoughi, M.; Esmaeili, E. Effective surface modification of MnFe2O4 @SiO2 @PMIDA magnetic nanoparticles for rapid and high-density antibody immobilization. Appl. Surf. Sci. 2017, 426, 1023–1029. [Google Scholar] [CrossRef]
- Huang, F.; Guo, R.; Xue, L.; Cai, G.; Wang, S.; Li, Y.; Liao, M.; Wang, M.; Lin, J. An Acid-Responsive Microfluidic Salmonella Biosensor Using Curcumin as Signal Reporter and ZnO-Capped Mesoporous Silica Nanoparticles for Signal Amplification. Sens. Actuators B Chem. 2020, 312, 127958. [Google Scholar] [CrossRef]
- Ghodsi, J.; Hajian, A.; Rafati, A.A.; Shoja, Y.; Yurchenko, O.; Urban, G. Electrostatically immobilized hemoglobin on silica-coated magnetic nanoparticles for simultaneous determination of dopamine, uric acid, and folic acid. J. Electrochem. Soc. 2016, 163, B609. [Google Scholar] [CrossRef]
- Achadu, O.J.; Nwaji, N.; Lee, D.; Lee, J.; Akinoglu, E.M.; Giersig, M.; Park, E.Y. 3D hierarchically porous magnetic molybdenum trioxide@gold nanospheres as a nanogap-enhanced Raman scattering biosensor for SARS-CoV-2. Nanoscale Adv. 2022, 4, 871–883. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, S.E.; Hong, J.S.; Im, H.; Hwang, S.Y.; Oh, J.K.; Kim, S.E. Gold-Nanoparticle-Coated Magnetic Beads for ALP-Enzyme-Based Electrochemical Immunosensing in Human Plasma. Materials 2022, 15, 6875. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 2017, 114, 24–32. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Wei, W.; Xia, N.; Du, Y. Magnetic Fe3O4-Based Sandwich-Type Biosensor Using Modified Gold Nanoparticles as Colorimetric Probes for the Detection of Dopamine. Materials 2013, 6, 5690–5699. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cui, J.; Wang, Y.; Jia, J. An ultrasensitive electrochemical biosensor for bisphenol A based on aptamer-modified MrGO@AuNPs and ssDNA-functionalized AuNP@MBs synergistic amplification. Chemosphere 2022, 311, 137154. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xie, M.; Hong, F.; Chai, X.; Mi, H.; Zhou, X.; Fan, L.; Zhang, Q.; Ngai, T.; Liu, J. A Highly Sensitive Glucose Biosensor Based on Gold Nanoparticles/Bovine Serum Albumin/Fe3O4 Biocomposite Nanoparticles. Electrochim. Acta 2016, 222, 1709–1715. [Google Scholar] [CrossRef]
- Della Ventura, B.; Campanile, R.; Cimafonte, M.; Elia, V.; Forente, A.; Minopoli, A.; Scardapane, E.; Schiattarella, C.; Velotta, R. Gold Coated Nanoparticles Functionalized by Photochemical Immobilization Technique for Immunosensing. In Sensors and Microsystems. AISEM 2020. Lecture Notes in Electrical Engineering; Springer: Cham, Switzerland, 2021; Volume 753, pp. 113–118. [Google Scholar]
- Weng, C.; Li, X.; Lu, Q.; Yang, W.; Wang, J.; Yan, X.; Li, B.; Sakran, M.; Hong, J.; Zhu, W.; et al. A label-free electrochemical biosensor based on magnetic biocomposites with DNAzyme and hybridization chain reaction dual signal amplification for the determination of Pb(2). Mikrochim. Acta 2020, 187, 575. [Google Scholar] [CrossRef]
- Choi, G.; Kim, E.; Park, E.; Lee, J.H. A cost-effective chemiluminescent biosensor capable of early diagnosing cancer using a combination of magnetic beads and platinum nanoparticles. Talanta 2017, 162, 38–45. [Google Scholar] [CrossRef]
- MKim, S.; Kweon, S.H.; Cho, S.; An, S.S.A.; Kim, M.I.; Doh, J.; Lee, J. Pt-Decorated Magnetic Nanozymes for Facile and Sensitive Point-of-Care Bioassay. ACS Appl. Mater. Interfaces 2017, 9, 35133–35140. [Google Scholar]
- Borisova, B.; Sánchez, A.; Jiménez-Falcao, S.; Martín, M.; Salazar, P.; Parrado, C.; Pingarrón, J.M.; Villalonga, R. Reduced graphene oxide-carboxymethylcellulose layered with platinum nanoparticles/PAMAM dendrimer/magnetic nanoparticles hybrids. Application to the preparation of enzyme electrochemical biosensors. Sens. Actuators B Chem. 2016, 232, 84–90. [Google Scholar] [CrossRef]
- Šafranko, S.; Goman, D.; Stanković, A.; Medvidović-Kosanović, M.; Moslavac, T.; Jerković, I.; Jokić, S. An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio) Molecule Sensing. Chemosensors 2021, 9, 138. [Google Scholar] [CrossRef]
- Pandit, C.; Alajangi, H.K.; Singh, J.; Khajuria, A.; Sharma, A.; Hassan, M.S.; Parida, M.; Semwal, A.D.; Gopalan, N.; Sharma, R.K.; et al. Development of magnetic nanoparticle assisted aptamer-quantum dot based biosensor for the detection of Escherichia coli in water samples. Sci. Total Environ. 2022, 831, 154857. [Google Scholar] [CrossRef]
- Shi, R.; Feng, S.; Park, C.Y.; Park, K.Y.; Song, J.; Park, J.P.; Chun, H.S.; Park, T.J. Fluorescence detection of histamine based on specific binding bioreceptors and carbon quantum dots. Biosens. Bioelectron. 2020, 167, 112519. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carinelli, S.; Luis-Sunga, M.; González-Mora, J.L.; Salazar-Carballo, P.A. Synthesis and Modification of Magnetic Nanoparticles for Biosensing and Bioassay Applications: A Review. Chemosensors 2023, 11, 533. https://doi.org/10.3390/chemosensors11100533
Carinelli S, Luis-Sunga M, González-Mora JL, Salazar-Carballo PA. Synthesis and Modification of Magnetic Nanoparticles for Biosensing and Bioassay Applications: A Review. Chemosensors. 2023; 11(10):533. https://doi.org/10.3390/chemosensors11100533
Chicago/Turabian StyleCarinelli, Soledad, Maximina Luis-Sunga, José Luis González-Mora, and Pedro A. Salazar-Carballo. 2023. "Synthesis and Modification of Magnetic Nanoparticles for Biosensing and Bioassay Applications: A Review" Chemosensors 11, no. 10: 533. https://doi.org/10.3390/chemosensors11100533
APA StyleCarinelli, S., Luis-Sunga, M., González-Mora, J. L., & Salazar-Carballo, P. A. (2023). Synthesis and Modification of Magnetic Nanoparticles for Biosensing and Bioassay Applications: A Review. Chemosensors, 11(10), 533. https://doi.org/10.3390/chemosensors11100533