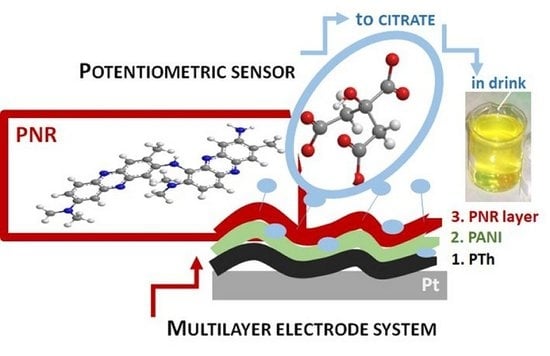
The Novel Three-Layer Electrode Based on Poly(Neutral Red) for Potentiometric Determination of Citrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Electrode Systems
2.3. Spectroscopic Characterization
2.4. Potentiometric Properties of Multilayer Polymer Electrodes
2.4.1. Calibration, Selectivity, and Stability
2.4.2. Real-World Sample Analysis
3. Results and Discussion
3.1. Polymerized Multilayer Electrode Preparation by Cyclic Voltammetry
3.2. Characterization of Multilayer Polymer Systems
3.2.1. Raman Spectroscopy
3.2.2. Electron Microscopy
3.2.3. UV–Vis Spectrometry
3.3. Potentiometry
3.3.1. Potentiometric Response to Citrate
3.3.2. Potentiometric Selectivity
3.3.3. Electrode Response and Stability
3.3.4. Potentiometric Determination of Citrate in Soft Drinks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukas, C.A.; Markovic, N.M.; Ross, P.N. Adsorption of halide anions at the Pt(111)-solution interface studied by in situ surface x-ray scattering. Phys. Rev. B Condens. Matter 1997, 55, 7964. [Google Scholar] [CrossRef]
- Simonet, J. Cathodic reactivity of platinum interface in the presence of tetramethylammonium salts. A pro-base cathode material? Electrochem. Commun. 2003, 5, 439–444. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Broncová, G.; Krondak, M.; Sýkora, D.; Král, V. Important aspects influencing stability of the electrochemical potential of conductive polymer-based electrodes. J. Mater. Sci. 2011, 46, 1–9. [Google Scholar] [CrossRef]
- Alumaa, A.; Hallik, A.; Mäeorg, U.; Sammelselg, V.; Tamm, J. Potentiometric properties of polypyrrole bilayers. Electrochim. Acta 2004, 49, 1767–1774. [Google Scholar] [CrossRef]
- Broncová, G.; Shishkanova, T.V.; Matějka, P.; Kubáč, D.; Král, V. Poly(Neutral Red) in Multilayer Electrode Systems. Chem. Listy 2016, 110, 800–807. [Google Scholar]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors Based on Conducting Polymers. Electroanalysis 2003, 15, 366–374. [Google Scholar] [CrossRef]
- Broncova, G.; Shishkanova, T.V.; Matejka, P.; Volf, R.; Král, V. Citrate selectivity of poly(neutral red) electropolymerized films. Anal. Chim. Acta 2004, 511, 197–205. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting Polymers—A New Era in Electrochemistry, 2nd ed.; Springer: New York, NY, USA, 2012; ISBN 978-3-642-27620-0. [Google Scholar] [CrossRef]
- Broncová, G.; Shishkanova, T.V.; Krondak, M.; Volf, R.; Král, V. Potentiometric Sensors Based on Conducting Polymers: Preparation, Response Mechanisms and Applications. Chem. Listy 2009, 103, 795–799. [Google Scholar]
- Bobacka, J.; Gao, Z.; Ivaska, A. Electrochemical study on polypyrrole—poly(3-octylthiophene) bilayer films. Synth. Met. 1993, 55, 1453–1458. [Google Scholar] [CrossRef]
- Gao, Z.; Bobacka, J.; Ivaska, A. Electrochemical study of bilayer conducting polymers: Polypyrrole/polyaniline system. J. Electroanal. Chem. 1994, 364, 127–133. [Google Scholar] [CrossRef]
- Wojda, A.; Maksymiuk, K. Electrochemical properties of bilayers of conducting polymers: Polypyrrole with poly(4-styrenesulfonate) ions/poly( N-methylpyrrole). Studies of the permeability of the outer layer towards cations. J. Electroanal. Chem. 1997, 424, 93–99. [Google Scholar] [CrossRef]
- Sari, B.; Talu, M. Electrochemical copolymerization of pyrrole and aniline. Synth. Met. 1998, 94, 221–227. [Google Scholar] [CrossRef]
- Koncki, R.; Wolfbeis, O.S. Composite Films of Prussian Blue and N-Substituted Polypyrroles: Fabrication and Application to Optical Determination of pH. Anal. Chem. 1998, 70, 2544–2550. [Google Scholar] [CrossRef]
- Talu, M.; Kabasakaloglu, M.; Yildirim, F.; Sari, B. Electrochemical synthesis and characterization of homopolymers of polyfuran and polythiophene and bipolymer films polyfuran/polythiophene and polythiophene/polyfuran. Appl. Surf. Sci. 2001, 181, 51–60. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Sapurina, I.; Stejskal, J.; Král, V.; Volf, R. Ion-selective electrodes: Polyaniline modification and anion recognition. Anal. Chim. Acta 2005, 553, 160–168. [Google Scholar] [CrossRef]
- Broncová, G.; Shishkanova, T.V.; Krondak, M.; Volf, R.; Král, V. Optimalization of Poly(neutral red) Coated-wire Electrode for Determination of Citrate in Soft Drinks. Sensors 2008, 8, 594–606. [Google Scholar] [CrossRef] [Green Version]
- Baluchová, S.; Barek, J.; Tomé, L.I.N.; Brett, C.M.A.; Schwarzová-Pecková, K. Vanillylmandelic and Homovanillic acid: Electroanalysis at non-modified and polymer-modified carbon-based electrodes. J. Electroanal. Chem. 2018, 821, 22–32. [Google Scholar] [CrossRef]
- Leidner, C.R.; Denisevich, P.; Willman, K.W.; Murray, R.W. Charge trapping reactions in bilayer electrodes. J. Electroanal. Chem. 1984, 164, 63–78. [Google Scholar] [CrossRef]
- Ren, X.; Pickup, P.G. Impedance of Polypyrrole Perchlorate/Polypyrrole Poly(styrenesulfonate) Bilayer. J. Phys. Chem. 1993, 97, 3941–3943. [Google Scholar] [CrossRef]
- Wojda, A.; Maksymiuk, K. Potentiometric studies of bilayers of conducting polymers. J. Electroanal. Chem. 1998, 441, 205–214. [Google Scholar] [CrossRef]
- Mangold, K.-M.; Schäfer, S.; Jüttner, K. Reference electrodes based on conducting polymer bilayers. Synth. Met. 2001, 119, 345–346. [Google Scholar] [CrossRef]
- Weng, S.; Zhou, J.; Lin, Z. Preparation of one-dimensional (1D) polyaniline–polypyrrole coaxial nanofibers and their application in gas sensor. Synth. Met. 2010, 160, 1136–1142. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M.; Šeděnková, I.; Kovářová, J.; Kopecká, J.; Prokeš, J. Coaxial conducting polymer nanotubes: Polypyrrole nanotubes coated with polyaniline or poly(p-phenylenediamine) and products of their carbonization. Chem. Pap. 2015, 69, 1341–1349. [Google Scholar] [CrossRef]
- Valaski, R.; Ayoub, S.; Micaroni, L.; Hümmelgen, I.A. Polypyrrole-poly(3-methylthiophene) bilayer films electrochemically deposited onto tin oxide. J. Solid State Electrochem. 2002, 6, 231–236. [Google Scholar] [CrossRef]
- Volf, R.; Král, V.; Hrdlička, J.; Shishkanova, T.V.; Broncová, G.; Krondak, M.; Stastný, M.; Kroulík, J.; Valík, M.; Matějka, P.; et al. Preparation, characterization and analytical application of electropolymerized films. Solid State Ionics 2002, 57, 154–155. [Google Scholar] [CrossRef]
- Záruba, K.; Matějka, P.; Volf, R.; Volka, K.; Král, V.; Sessler, J.L. Formation of Porphyrin- and Sapphyrin-Containing Monolayers on Electrochemically Prepared Gold Substrates: A FT Raman Spectroscopic Study. Langmuir 2002, 18, 6896–6906. [Google Scholar] [CrossRef]
- Březnová, H.; Volf, R.; Král, V.; Sessler, J.L.; Try, A.C.; Shishkanova, T.V. Monomer and polymer quinoxaline derivatives for cationic recognition. Anal. Bioanal. Chem. 2003, 375, 1193–1198. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Koryta, J.; Štulík, K. Iontově-Selektivní Elektrody (Ion-Selective Electrode); Academia: Prague, Czech, 1984; ISBN 21-035-84. [Google Scholar]
- Udum, A.Y.; Pekmez, K.; Yildiz, A. Electropolymerization of self-doped polythiophene in acetonitrile containing FSO3H. Synth. Met. 2004, 142, 7–12. [Google Scholar] [CrossRef]
- Choi, S.-J.; Park, S.-M. Electrochemistry of Conductive Polymers. XXVI. Effects of Electrolytes and Growth Methods on Polyaniline Morphology. J. Electrochem. Soc. 2002, 149, E26–E34. [Google Scholar] [CrossRef]
- Pandey, P.C.; Singh, G. Tetraphenylborate doped polyaniline based novel pH sensor and solid-state urea biosensor. Talanta 2001, 55, 773–782. [Google Scholar] [CrossRef]
- Sulimenko, T.; Stejskal, J.; Křivka, I.; Prokeš, J. Conductivity of colloidal polyaniline dispersions. Eur. Polym. J. 2001, 37, 219–226. [Google Scholar] [CrossRef]
- He, J.; Zhou, H.; Wan, F.; Lu, F.; Xue, G. SERS study of the high quality conducting polythiophene film. Vib. Spectrosc. 2003, 31, 265–269. [Google Scholar] [CrossRef]
- Karakisla, M.; Aksu, L.; Sacak, M. Polypyrrole/polyaniline conductive films obtained electrochemically on polycarbonate-coated platinum electrodes. Polym. Int. 2002, 51, 1371–1377. [Google Scholar] [CrossRef]
- Berrada, K.; Quillard, S.; Louam, G.; Lefrant, S. Polyanilines and substituted polyanilines: A comparative study of the Raman spectra of leucoemeraldine, emeraldine and pernigraniline. Synth. Met. 1995, 69, 201–204. [Google Scholar] [CrossRef]
- Baibarac, M.; Cochet, M.; Lapkowski, M.; Mihut, L.; Lefrant, S.; Baltog, I. SERS spectra of polyaniline thin films deposited on rough Ag, Au and Cu. Polymer film thickness and roughness parameter dependence of SERS spectra. Synth. Met. 1998, 96, 63–70. [Google Scholar] [CrossRef]
- Benito, D.; Gabrielli, C.; García-Jareño, J.J.; Keddam, M.; Perrot, H.; Vicente, F. An electrochemical impedance and ac-electrogravimetry study of PNR films in aqueous salt media. Electrochem. Commun. 2002, 4, 613–619. [Google Scholar] [CrossRef]
- Saez, E.I.; Corn, R.M. In situ polarization modulation—Fourier transform infrared spectroelectrochemistry of phenazine and phenothiazine dye films at polycrystalline gold electrodes. Electrochim. Acta 1993, 38, 1619–1625. [Google Scholar] [CrossRef]
- Schlereth, D.D.; Karyakin, A.A. Electropolymerization of phenothiazine, phenoxazine and phenazine derivatives: Characterization of the polymers by UV-visible difference spectroelectrochemistry and Fourier transform IR spectroscopy. J. Electroanal. Chem. 1995, 395, 221–232. [Google Scholar] [CrossRef]
- Lindner, E.; Umeyawa, Y. Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC Technical Report) Pure. Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Response mechanism of potentiometric Ag+ sensor based on PEDOT doped with silver hexabromocarborane. J. Electroanal. Chem. 2006, 593, 219–226. [Google Scholar] [CrossRef]
- Wiskur, S.L.; Ait-Haddou, H.; Lavigne, J.J.; Anslyn, E.V. Teaching Old Indicators New Tricks. Acc. Chem. Res. 2001, 34, 963–972. [Google Scholar] [CrossRef] [PubMed]








| Composition of Polymerization Bath | Polymerization Parameters | |||
|---|---|---|---|---|
| Monomer | Monomer concentration (M) | Supporting electrolyte | Potential limits vs. Ag/AgCl (V) | Time of polymerization (min) |
| Thiophene | 0.4 | Acetonitrile 0.05 M TBAP | −0.20–1.90 | 4 |
| Aniline.H2SO4 | 0.05 | 1 M H2SO4 60 μM PVP | 0.00–1.20 | 10 |
| Neutral red [7] | 0.005 | Acetonitrile 0.05 M TBAP | −0.20–1.80 | 15 |
| Form | Maximum I (nm) | Maximum II (nm) |
|---|---|---|
| Monomer | 456 | 541 |
| Polymer | 449 | 537 |
| pH | Monomer Max (nm) | Polymer Max (nm) |
|---|---|---|
| 4 | 530 | 531 |
| 5 | 529 | 529 |
| 6 | 529 | 445 + sh * (530) |
| 7 | 461 + sh * (530) | 443 |
| 8 | 453 | 442 |
| Parameters | PNR | PTh | PANI | PTh-PNR | PTh-PANI- PNR | PTh-PNR- PANI |
|---|---|---|---|---|---|---|
| Slope S (mV decade−1) | −22.7 | −53.5 | −20.4 | −25.4 | −18.0 | −19.5 |
| Conc. range (M) | 10−5–10−1 | 10−2–10−1 | 10−3–10−1 | 10−5–10−1 | 10−6–10−1 | 10−4–10−1 |
| Coefficient of determination R2 | 0.9927 | 1 | 0.9983 | 0.9814 | 0.9895 | 0.9893 |
| DL * (M) | 2.4 × 10−6 | - | 1.0 × 10−4 | 1.5 × 10−6 | 4.3 × 10−6 | 2.0 × 10−6 |
| Sample | PTh-PANI-PNR Electrodes | PNR (org) Electrodes from the Reference | Another Method (CE) [17] | Another Method (Abs) [44] |
|---|---|---|---|---|
| Mountain Dew | 1.56 ± 0.13 * | 1.74 ± 0.27 * | 1.37 ± 0.08 * | 1.42 |
| Gatorade | 3.18 ± 0.21 * | 3.01 ± 0.25 * | 3.43 ± 0.19 * | 3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broncová, G.; Shishkanova, T.V.; Matějka, P. The Novel Three-Layer Electrode Based on Poly(Neutral Red) for Potentiometric Determination of Citrates. Chemosensors 2023, 11, 170. https://doi.org/10.3390/chemosensors11030170
Broncová G, Shishkanova TV, Matějka P. The Novel Three-Layer Electrode Based on Poly(Neutral Red) for Potentiometric Determination of Citrates. Chemosensors. 2023; 11(3):170. https://doi.org/10.3390/chemosensors11030170
Chicago/Turabian StyleBroncová, Gabriela, Tatiana V. Shishkanova, and Pavel Matějka. 2023. "The Novel Three-Layer Electrode Based on Poly(Neutral Red) for Potentiometric Determination of Citrates" Chemosensors 11, no. 3: 170. https://doi.org/10.3390/chemosensors11030170
APA StyleBroncová, G., Shishkanova, T. V., & Matějka, P. (2023). The Novel Three-Layer Electrode Based on Poly(Neutral Red) for Potentiometric Determination of Citrates. Chemosensors, 11(3), 170. https://doi.org/10.3390/chemosensors11030170