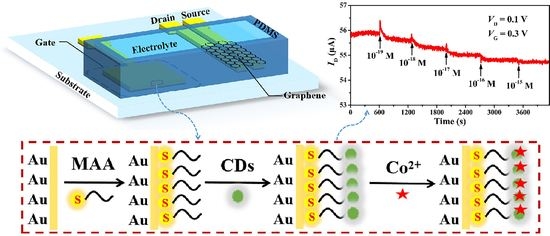
Carbon Dot-Functionalized Solution-Gated Graphene Transistors for Highly Sensitive Detection of Cobalt(II) Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CDs
2.3. Device Fabrication
2.4. Gate Electrode Modification with CDs
2.5. Device Test
3. Results and Discussion
3.1. Synthesis and Characterization of CDs
3.2. Detection of Co2+ Ions Using CD-SGGT
3.3. Fluorescence Detection of Co2+ Ions Using CDs
3.4. The Sensing Mechanism of CD−SGGT for Co2+ Ions Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vashisht, D.; Kaur, K.; Jukaria, R.; Vashisht, A.; Sharma, S.; Mehta, S.K. Colorimetric chemosensor based on coumarin skeleton for selective naked eye detection of cobalt (II) ion in near aqueous medium. Sens. Actuators B Chem. 2019, 280, 219–226. [Google Scholar] [CrossRef]
- Russell, M.J. Cobalt: A must-have element for life and livelihood. Proc. Natl. Acad. Sci. USA 2022, 119, 2121307119. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Ismael, M.; Yaita, T. Efficient detection and extraction of cobalt(II) from lithium ion batteries and wastewater by novel composite adsorbent. Sens. Actuators B Chem. 2014, 191, 9–18. [Google Scholar] [CrossRef]
- Hu, G.; Ge, L.; Li, Y.; Mukhtar, M.; Shen, B.; Yang, D.; Li, J. Carbon dots derived from flax straw for highly sensitive and selective detections of cobalt, chromium, and ascorbic acid. J. Colloid Interface Sci. 2020, 579, 96–108. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Boonta, W.; Talodthaisong, C.; Sattayaporn, S.; Chaicham, C.; Chaicham, A.; Sahasithiwat, S.; Kangkaew, L.; Kulchat, S. The synthesis of nitrogen and sulfur co-doped graphene quantum dots for fluorescence detection of cobalt(ii) ions in water. Mater. Chem. Front. 2020, 4, 507–516. [Google Scholar] [CrossRef]
- Du, F.; Cheng, Z.; Kremer, M.; Liu, Y.; Wang, X.; Shuang, S.; Dong, C. A label-free multifunctional nanosensor based on N-doped carbon nanodots for vitamin B12 and Co2+ detection, and bioimaging in living cells and zebrafish. J. Mater. Chem. B 2020, 8, 5089–5095. [Google Scholar] [CrossRef]
- He, Z.; Zhu, J.; Li, X.; Weng, G.J.; Li, J.J.; Zhao, J.W. Surface etching-dependent geometry tailoring and multi-spectral information of Au@AuAg yolk-shell nanostructure with asymmetrical pyramidal core: The application in Co2+ determination. J. Colloid Interface Sci. 2022, 625, 340–353. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Dige, N.C.; Desai, N.K.; Patil, S.R.; Kondalkar, V.V.; Hong, S.K.; Lee, K.H. Selective detection of Co2+ by fluorescent nano probe: Diagnostic approach for analysis of environmental samples and biological activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 198, 136–144. [Google Scholar] [CrossRef]
- Tsoutsi, D.; Guerrini, L.; Hermida-Ramon, J.M.; Giannini, V.; Liz-Marzan, L.M.; Wei, A.; Alvarez-Puebla, R.A. Simultaneous SERS detection of copper and cobalt at ultratrace levels. Nanoscale 2013, 5, 5841–5846. [Google Scholar] [CrossRef]
- Shi, J.; Lu, C.; Yan, D.; Ma, L. High selectivity sensing of cobalt in HepG2 cells based on necklace model microenvironment-modulated carbon dot-improved chemiluminescence in Fenton-like system. Biosens. Bioelectron. 2013, 45, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Hu, B. Thermo-responsive polymer coated fiber-in-tube capillary microextraction and its application to on-line determination of Co, Ni and Cd by inductively coupled plasma mass spectrometry (ICP-MS). Talanta 2011, 85, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Asiri, A.M.; Arshad, M.N.; Rahman, M.M. Development of selective Co2+ ionic sensor based on various derivatives of benzenesulfonohydrazide (BSH) compound: An electrochemical approach. Chem. Eng. J. 2018, 339, 133–143. [Google Scholar] [CrossRef]
- Lee, H.; Sung, H.K.; Park, C.; Kim, Y. Bimetallic Au/Ag nanoframes as spectator for Co2+ ion. J. Ind. Eng. Chem. 2017, 48, 235–241. [Google Scholar] [CrossRef]
- Mehta, V.N.; Desai, M.L.; Basu, H.; Kumar Singhal, R.; Kailasa, S.K. Recent developments on fluorescent hybrid nanomaterials for metal ions sensing and bioimaging applications: A review. J. Mol. Liq. 2021, 333, 115950. [Google Scholar] [CrossRef]
- Liu, Z.; Pickens Iii, D.; He, T.; Zhang, X.; Liu, Y.; Nishino, T.; Jane Wang, Q. A Thermal Elastohydrodynamic Lubrication Model for Crowned Rollers and Its Application on Apex Seal–Housing Interfaces. J. Tribol. 2019, 141, 041501. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Fu, Z.; Gao, W.; Zhang, C.; Zhao, Q.; Deng, F.; Lu, X. Review on carbon dots in food safety applications. Talanta 2019, 194, 809–821. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Saucedo, N.M.; Gao, J.; Li, X.; Mulchandani, A. An electrochemically reduced graphene oxide chemiresistive sensor for sensitive detection of Hg2+ ion in water samples. J. Hazard. Mater. 2016, 320, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Zhang, T.; Kong, W.; Zhang, Z.; Qu, H.; Chen, W.; Wang, Y.; Luo, L.; Zheng, L. ZIF-67 derived porous Co3O4 hollow nanopolyhedron functionalized solution-gated graphene transistors for simultaneous detection of glucose and uric acid in tears. Biosens. Bioelectron. 2018, 101, 21–28. [Google Scholar] [CrossRef]
- Kwon, S.S.; Yi, J.; Lee, W.W.; Shin, J.H.; Kim, S.H.; Cho, S.H.; Nam, S.; Park, W.I. Reversible and Irreversible Responses of Defect-Engineered Graphene-Based Electrolyte-Gated pH Sensors. ACS Appl. Mater. 2016, 8, 834–839. [Google Scholar] [CrossRef]
- Zou, H.L.; Li, B.L.; Luo, H.Q.; Li, N.B. A novel electrochemical biosensor based on hemin functionalized graphene oxide sheets for simultaneous determination of ascorbic acid, dopamine and uric acid. Sen. Actuators B Chem. 2015, 207, 535–541. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, N.; Yang, A.; Law, H.K.; Li, L.; Yan, F. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Loan, P.T.K.; Chen, T.-Y.; Liu, K.-K.; Chen, C.-H.; Wei, K.-H.; Li, L.-J. Label-Free Electrical Detection of DNA Hybridization on Graphene using Hall Effect Measurements: Revisiting the Sensing Mechanism. Adv. Funct. 2013, 23, 2301–2307. [Google Scholar] [CrossRef]
- Hess, L.H.; Jansen, M.; Maybeck, V.; Hauf, M.V.; Seifert, M.; Stutzmann, M.; Sharp, I.D.; Offenhausser, A.; Garrido, J.A. Graphene transistor arrays for recording action potentials from electrogenic cells. Adv. Mater. 2011, 23, 5045–5049. [Google Scholar] [CrossRef]
- Park, S.; Choi, J.; Jeun, M.; Kim, Y.; Yuk, S.S.; Kim, S.K.; Song, C.S.; Lee, S.; Lee, K.H. Detection of Avian Influenza Virus from Cloacal Swabs Using a Disposable Well Gate FET Sensor. Adv. Healthc. Mater. 2017, 6, 1700371. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Shi, Y.; Li, C.M.; Li, L.J.; Chen, P. Nanoelectronic biosensors based on CVD grown graphene. Nanoscale 2010, 2, 1485–1488. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Stine, R.; Mulvaney, S.P.; Robinson, J.T.; Tamanaha, C.R.; Sheehan, P.E. Fabrication, optimization, and use of graphene field effect sensors. Anal. Chem. 2013, 85, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Wang, L.; Xu, D.; Duo, Y.; Gao, J.; Zhang, L.; Wang, X.; Chen, X.; Li, J.; Zhang, H. Solution-gated transistors of two-dimensional materials for chemical and biological sensors: Status and challenges. Nanoscale 2020, 12, 11364–11394. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, M.; Li, J. Solution-gated graphene transistors for chemical and biological sensors. Adv. Healthc. Mater. 2014, 3, 313–331. [Google Scholar] [CrossRef]
- Fan, Q.; Li, J.; Wang, J.; Yang, Z.; Shen, T.; Guo, Y.; Wang, L.; Irshad, M.S.; Mei, T.; Wang, X. Ultrasensitive Fe3+ ion detection based on carbon quantum dot-functionalized solution-gated graphene transistors. J. Mater. Chem. C 2020, 8, 4685–4689. [Google Scholar] [CrossRef]
- Fan, Q.; Li, J.; Zhu, Y.; Yang, Z.; Shen, T.; Guo, Y.; Wang, L.; Mei, T.; Wang, J.; Wang, X. Functional Carbon Quantum Dots for Highly Sensitive Graphene Transistors for Cu2+ Ion Detection. ACS Appl. Mater. 2020, 12, 4797–4803. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhu, B.; Wang, Y.; Wu, H.; Chai, F.; Qu, F.; Su, Z. Nitrogen- and sulfur-doped carbon dots as peroxidase mimetics: Colorimetric determination of hydrogen peroxide and glutathione, and fluorimetric determination of lead(II). Mikrochim. Acta 2019, 186, 604. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, X.; Zhang, Z.; Kang, D.; Zhang, J.; Liu, K.; Li, X.; Shen, J.; Liu, F.; Wang, T.; et al. Epitaxy of Single-Crystalline GaN Film on CMOS-Compatible Si(100) Substrate Buffered by Graphene. Adv. Funct. 2019, 29, 1905056. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.Z.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.Y.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Micropor. Mesopor. Mat. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Li, J.; Xu, L.; Qiao, Y. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J. Colloid Interface Sci. 2017, 485, 167–174. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, F.; Li, Y. Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sens. Actuators B Chem. 2018, 255, 1105–1111. [Google Scholar] [CrossRef]
- Wang, B.; Huang, M.; Kim, N.Y.; Cunning, B.V.; Huang, Y.; Qu, D.; Chen, X.; Jin, S.; Biswal, M.; Zhang, X.; et al. Controlled Folding of Single Crystal Graphene. Nano Lett. 2017, 17, 1467–1473. [Google Scholar] [CrossRef]
- Song, Y.; Zou, W.; Lu, Q.; Lin, L.; Liu, Z. Graphene Transfer: Paving the Road for Applications of Chemical Vapor Deposition Graphene. Small 2021, 17, 2007600. [Google Scholar] [CrossRef]
- Gao, Y.; Jiao, Y.; Lu, W.; Liu, Y.; Han, H.; Gong, X.; Xian, M.; Shuang, S.; Dong, C. Carbon dots with red emission as a fluorescent and colorimeteric dual-readout probe for the detection of chromium(vi) and cysteine and its logic gate operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al(iii)/In(iii)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.-Q.; Kumar Singh, A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Wang, J.; Xue, C.; Deng, M.; Li, Z.; Zhang, H.; Cai, C.; Xu, B.; Wang, X.; Li, J. Carbon Dot-Functionalized Solution-Gated Graphene Transistors for Highly Sensitive Detection of Cobalt(II) Ions. Chemosensors 2023, 11, 192. https://doi.org/10.3390/chemosensors11030192
Ren Z, Wang J, Xue C, Deng M, Li Z, Zhang H, Cai C, Xu B, Wang X, Li J. Carbon Dot-Functionalized Solution-Gated Graphene Transistors for Highly Sensitive Detection of Cobalt(II) Ions. Chemosensors. 2023; 11(3):192. https://doi.org/10.3390/chemosensors11030192
Chicago/Turabian StyleRen, Zhanpeng, Jianying Wang, Chenglong Xue, Minghua Deng, Ziqin Li, Huibin Zhang, Chen Cai, Bing Xu, Xianbao Wang, and Jinhua Li. 2023. "Carbon Dot-Functionalized Solution-Gated Graphene Transistors for Highly Sensitive Detection of Cobalt(II) Ions" Chemosensors 11, no. 3: 192. https://doi.org/10.3390/chemosensors11030192
APA StyleRen, Z., Wang, J., Xue, C., Deng, M., Li, Z., Zhang, H., Cai, C., Xu, B., Wang, X., & Li, J. (2023). Carbon Dot-Functionalized Solution-Gated Graphene Transistors for Highly Sensitive Detection of Cobalt(II) Ions. Chemosensors, 11(3), 192. https://doi.org/10.3390/chemosensors11030192