In-Situ Formation of NiFe-MOF on Nickel Foam as a Self-Supporting Electrode for Flexible Electrochemical Sensing and Energy Conversion
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Medicines
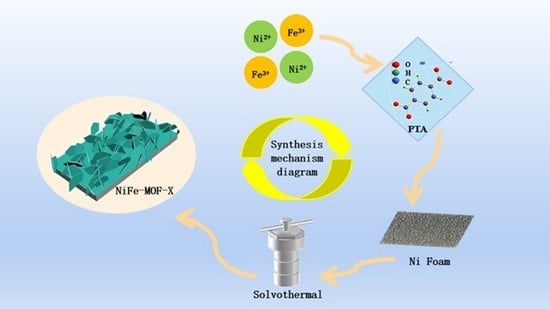
2.2. Synthesis of NiFe-MOF-X
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Conformational Characterization of NiFe-MOF-X
3.2. Structural Characterization of NiFe-MOF-X
3.3. Electrochemical Properties of NiFe-MOF-X
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.L.; Xu, Q. Metal-organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Karimi, B.; Aghdam, S.A.; Behzadnia, H.; Kiani, M.A. Polyaniline-ionic liquid derived ordered mesoporous carbon nanocomposite: Synthesis and supercapacitive behavior. RSC Adv. 2015, 5, 69032–69041. [Google Scholar] [CrossRef]
- Kishore babu, S.; Jayachandran, M.; Maiyalagan, T.; Vijayakumar, T.; Gunasekaran, B. Metal-organic framework (MOF-5) incorporated on NiCo2O4 as electrode material for supercapacitor application. Mater. Lett. 2021, 302, 130338. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Li, P.Y.; Jiao, Y.; Yao, S.Y.; Wang, L.X.; Chen, G. Dual role of nickel foam in NiCoAl-LDH ensuring high-performance for asymmetric supercapacitors. New J. Chem. 2019, 43, 3139–3145. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, Y.; Yu, M.; Liu, P.; Tong, Y.; Cheng, F.; Lu, X. Recent smart methods for achieving high-energy asymmetric supercapacitors. Small Methods 2018, 2, 1700230. [Google Scholar] [CrossRef]
- Yu, J.; Dai, Y.; He, Q.; Zhao, D.; Shao, Z.; Ni, M. A mini-review of noble-metal-free electrocatalysts for overall water splitting in non-alkaline electrolytes. Mater. Rep. Energy 2021, 1, 100024. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Xu, X.M.; Shao, Z.P.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, H.E.; Cho, A.; Kim, S.; Ye, Y.; Han, J.W.; Lee, H.; Jang, J.H.; Lee, J. Investigation of the support effect in atomically dispersed Pt on WO3-x for utilization of Pt in the hydrogen evolution reaction. Angew. Chem. Int. Ed. Engl. 2019, 58, 16038–16042. [Google Scholar] [CrossRef]
- Du, L.; Sun, Y.; You, B. Hybrid water electrolysis: Replacing oxygen evolution reaction for energy-efficient hydrogen production and beyond. Mater. Rep. Energy 2021, 1, 100004. [Google Scholar] [CrossRef]
- Urbain, F.; Du, R.; Tang, P.; Smirnov, V.; Andreu, T.; Finger, F.; Divins, N.J.; Llorca, J.; Arbiol, J.; Cabot, A.; et al. Upscaling high activity oxygen evolution catalysts based on CoFe2O4 nanoparticles supported on nickel foam for power-to-gas electrochemical conversion with energy efficiencies above 80%. Appl. Catal. B Environ. 2019, 259, 118055. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.Y.; Yan, C.S.; Chen, D.H.; Lv, C.D.; Jiao, Y.; Chen, G. One-dimensional Co3O4 nanonet with enhanced rate performance for lithium ion batteries: Carbonyl-beta-cyclodextrin inducing and kinetic analysis. Chem. Eng. J. 2017, 321, 31–39. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Hauser, J.L.; Chen, M.; Oliver, S.R.J.; Ye, Y.; Guo, J.; Li, Y. Ni foam-supported Fe-doped β-Ni(OH)2 nanosheets show ultralow overpotential for oxygen evolution reaction. ACS Energy Lett. 2019, 4, 622–628. [Google Scholar] [CrossRef]
- Xu, Y.C.; Jiao, Y.; Shen, L.G.; Chen, J.R.; Lin, H.J. Ultrathin graphene layer activated dendritic alpha-Fe2O3 for high performance asymmetric supercapacitors. J. Alloy Compd. 2019, 780, 212–219. [Google Scholar] [CrossRef]
- Deng, T.; Shi, X.; Zhang, W.; Wang, Z.; Zheng, W. Unlocking the potential of metal organic frameworks for synergized specific and areal capacitances via orientation regulation. Nanotechnology 2021, 32, 075402. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Zhu, Z.; Han, S.; Zhao, C.; Chen, G. One-pot construction of highly oriented Co-MOF nanoneedle arrays on Co foam for high-performance supercapacitor. Nanotechnology 2021, 32, 395606. [Google Scholar] [CrossRef]
- Chen, Q.; Lei, S.; Deng, P.; Ou, X.; Chen, L.; Wang, W.; Xiao, Y.; Cheng, B. Direct growth of nickel terephthalate on Ni foam with large mass-loading for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 19323–19332. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Zeng, Y.; Cheng, D.; Xiong, Y.; Bu, Y. Rational construction of triangle-like nickel-cobalt bimetallic metal-organic framework nanosheets arrays as battery-type electrodes for hybrid supercapacitors. J. Colloid Interface Sci. 2019, 555, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.; Sekhar, S.C.; Ramulu, B.; Hussain, S.K.; Narsimulu, D.; Yu, J.S. Ternary MOF-based redox active sites enabled 3D-on-2D nanoarchitectured battery-type electrodes for high-energy-density supercapatteries. Nanomicro. Lett. 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, X.; Yu, H.; Ding, T.; Yan, Y.; Yao, B.; Yao, X.; Chen, D.; Liu, M.; Huang, L. A Scalable Free-Standing V2O5/CNT Film Electrode for Supercapacitors with a Wide Operation Voltage (1.6 V) in an Aqueous Electrolyte. Adv. Funct. Mater. 2016, 26, 6114–6120. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.X.; Shao, F.Q.; Weng, S.T.; Xiong, S.S.; Liu, S.A.; Jiang, S.Y.; Xu, Y.C.; Jiao, Y.; Chen, J.R. Boosted charge transfer in oxygen vacancy-rich K+ birnessite MnO2 for water oxidation and zinc-ion batteries. Electrochem. Acta 2021, 378, 138147. [Google Scholar] [CrossRef]
- Yao, S.Y.; Jiao, Y.; Sun, S.F.; Wang, L.X.; Li, P.Y.; Chen, G. Vertically Co-oriented Mn-Metal-Organic Framework Grown on 2D Cation-Intercalated Manganese Oxide via a Self-sacrificing Template Process for a High-Performance Asymmetric Supercapacitor. ACS Sustain. Chem. Eng. 2018, 42, 4175–4181. [Google Scholar] [CrossRef]
- Youn, D.H.; Park, Y.B.; Kim, J.Y.; Magesh, G.; Jang, Y.J.; Lee, J.S. One-pot synthesis of NiFe layered double hydroxide/reduced graphene oxide composite as an efficient electrocatalyst for electrochemical and photoelectrochemical water oxidation. J. Power Sources 2015, 294, 437–443. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Li, X. Rational designing Ni3-xFexS2 nanosheet arrays on Ni foam to enhance supercapacitor performance. Ionics 2020, 26, 3677–3683. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Zhang, M.; Gong, Y. Vertically stacked bilayer heterostructure CoFe2O4@Ni3S2 on a 3D nickel foam as a high-performance electrocatalyst for the oxygen evolution reaction. New J. Chem. 2020, 44, 1455–1462. [Google Scholar] [CrossRef]
- Du, C.; Shang, M.; Mao, J.; Song, W. Hierarchical MoP/Ni2P heterostructures on nickel foam for efficient water splitting. J. Mater. Chem. A 2017, 5, 15940–15949. [Google Scholar] [CrossRef]
- Qu, S.; Huang, J.; Yu, J.; Chen, G.; Hu, W.; Yin, M.; Zhang, R.; Chu, S.; Li, C. Ni3S2 nanosheet flowers decorated with CdS quantum dots as a highly active electrocatalysis electrode for synergistic water splitting. ACS Appl. Mater. Interfaces 2017, 9, 29660–29668. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Prakasha, K.R.; Prakash, A.S.; Shim, J.J. Direct growth of honeycomb-like NiCo2O4@Ni foam electrode for pouch-type high-performance asymmetric supercapacitor. J. Alloys Compd. 2020, 836, 155370. [Google Scholar] [CrossRef]
- Xu, H.; Cao, Y.; Li, Y.; Cao, P.; Liu, D.; Zhang, Y.; Li, Q. High-loading Co-doped NiO nanosheets on carbon-welded carbon nanotube framework enabling rapid charge kinetic for enhanced supercapacitor performance. J. Energy Chem. 2020, 50, 240–247. [Google Scholar] [CrossRef]
- Hastak, R.S.; Sivaraman, P.; Potphode, D.D.; Shashidhara, K.; Samui, A.B. All solid supercapacitor based on activated carbon and poly [2, 5-benzimidazole] for high temperature application. Electrochim. Acta 2012, 59, 296–303. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, J.; Xia, W.; Peng, Z.; Wu, Z.; Lei, W.; Xia, K.; Xin, H.L.; Wang, D. Porous structured Ni–Fe–P nanocubes derived from a prussian blue analogue as an electrocatalyst for efficient overall water splitting. ACS Appl. Mater. Interfaces 2017, 9, 26134–26142. [Google Scholar] [CrossRef]
- Yuan, Q.; Yu, Y.; Gong, Y.; Bi, X. Three-dimensional N-doped carbon nanotube frameworks on Ni foam derived from a metal–organic framework as a bifunctional electrocatalyst for overall water splitting. ACS Appl. Mater. Interfaces 2019, 12, 3592–3602. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Simke, J.R.J.; Schmidt, J.; Thomas, A. Bifunctional Electrocatalysts for Overall Water Splitting from an Iron/Nickel-Based Bimetallic Metal-Organic Framework/Dicyandiamide Composite. Angew. Chem. Int. Ed. Engl. 2018, 57, 8921–8926. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, N.; Ren, L.; Casillas-Garcia, G.; Liu, N.; Liu, Y.; Xu, X.; Hao, W.; Dou, S.X.; Du, Y. In-situ grafting of N-doped carbon nanotubes with Ni encapsulation onto MOF-derived hierarchical hybrids for efficient electrocatalytic hydrogen evolution. Carbon 2020, 163, 178–185. [Google Scholar] [CrossRef]
- Qiao, H.; Yang, Y.; Dai, X.; Zhao, H.; Yong, J.; Yu, L.; Luan, X.; Cui, M.; Zhang, X.; Huang, X. Amorphous (Fe) Ni-MOF-derived hollow (bi) metal/oxide@ N-graphene polyhedron as effectively bifunctional catalysts in overall alkaline water splitting. Electrochim. Acta 2019, 318, 430–439. [Google Scholar] [CrossRef]
- Yu, J.; Lv, C.; Zhao, L.; Zhang, L.; Wang, Z.; Liu, Q. Reverse Microemulsion-Assisted Synthesis of NiCo2S4 Nanoflakes Supported on Nickel Foam for Electrochemical Overall Water Splitting. Adv. Mater. Interfaces 2018, 5, 1701396. [Google Scholar] [CrossRef]
- Sun, X.; Shao, Q.; Pi, Y.; Guo, J.; Huang, X. A general approach to synthesise ultrathin NiM (M = Fe, Co, Mn) hydroxide nanosheets as high-performance low-cost electrocatalysts for overall water splitting. J. Mater. Chem. A 2017, 5, 7769–7775. [Google Scholar] [CrossRef]
- Kong, F.; Fan, X.; Kong, A.; Zhou, Z.; Zhang, X.; Shan, Y. Covalent phenanthroline framework derived FeS@ Fe3C composite nanoparticles embedding in N-S-codoped carbons as highly efficient trifunctional electrocatalysts. Adv. Funct. Mater. 2018, 28, 1803973. [Google Scholar] [CrossRef]
- Dai, X.; Liu, M.; Li, Z.; Jin, A.; Ma, Y.; Huang, X.; Sun, H.; Wang, H.; Zhang, X. Molybdenum polysulfide anchored on porous Zr-metal organic framework to enhance the performance of hydrogen evolution reaction. J. Phys. Chem. C 2016, 120, 12539–12548. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Hercule, K.M.; Abruna, H.D.; Simon, P.; Dunn, B.; et al. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [Green Version]
- Askari, M.B.; Salarizadeh, P.; Beheshti-Marnani, A.; Di Bartolomeo, A. NiO-Co3O4-rGO as an efficient electrode material for supercapacitors and direct alcoholic fuel cells. Adv. Mater. Interfaces 2021, 8, 2100149. [Google Scholar] [CrossRef]
- Weng, Z.; Su, Y.; Wang, D.W.; Li, F.; Du, J.; Cheng, H.M. Graphene–cellulose paper flexible supercapacitors. Adv. Energy Mater. 2011, 1, 917–922. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, S.; An, Q.; Xu, Y.; Jiao, Y.; Chen, J. In-Situ Formation of NiFe-MOF on Nickel Foam as a Self-Supporting Electrode for Flexible Electrochemical Sensing and Energy Conversion. Chemosensors 2023, 11, 242. https://doi.org/10.3390/chemosensors11040242
Weng S, An Q, Xu Y, Jiao Y, Chen J. In-Situ Formation of NiFe-MOF on Nickel Foam as a Self-Supporting Electrode for Flexible Electrochemical Sensing and Energy Conversion. Chemosensors. 2023; 11(4):242. https://doi.org/10.3390/chemosensors11040242
Chicago/Turabian StyleWeng, Shuting, Qi An, Yanchao Xu, Yang Jiao, and Jianrong Chen. 2023. "In-Situ Formation of NiFe-MOF on Nickel Foam as a Self-Supporting Electrode for Flexible Electrochemical Sensing and Energy Conversion" Chemosensors 11, no. 4: 242. https://doi.org/10.3390/chemosensors11040242
APA StyleWeng, S., An, Q., Xu, Y., Jiao, Y., & Chen, J. (2023). In-Situ Formation of NiFe-MOF on Nickel Foam as a Self-Supporting Electrode for Flexible Electrochemical Sensing and Energy Conversion. Chemosensors, 11(4), 242. https://doi.org/10.3390/chemosensors11040242